Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Effective Synthesis of N,N-Diphenyl Carbazolium Salts
Synlett ( IF 2 ) Pub Date : 2017-12-20 , DOI: 10.1055/s-0036-1591848 Charles Diesendruck 1 , Sinai Aharonovich 1 , Nansi Gjineci 1 , Dario Dekel 2
Synlett ( IF 2 ) Pub Date : 2017-12-20 , DOI: 10.1055/s-0036-1591848 Charles Diesendruck 1 , Sinai Aharonovich 1 , Nansi Gjineci 1 , Dario Dekel 2
Affiliation
|
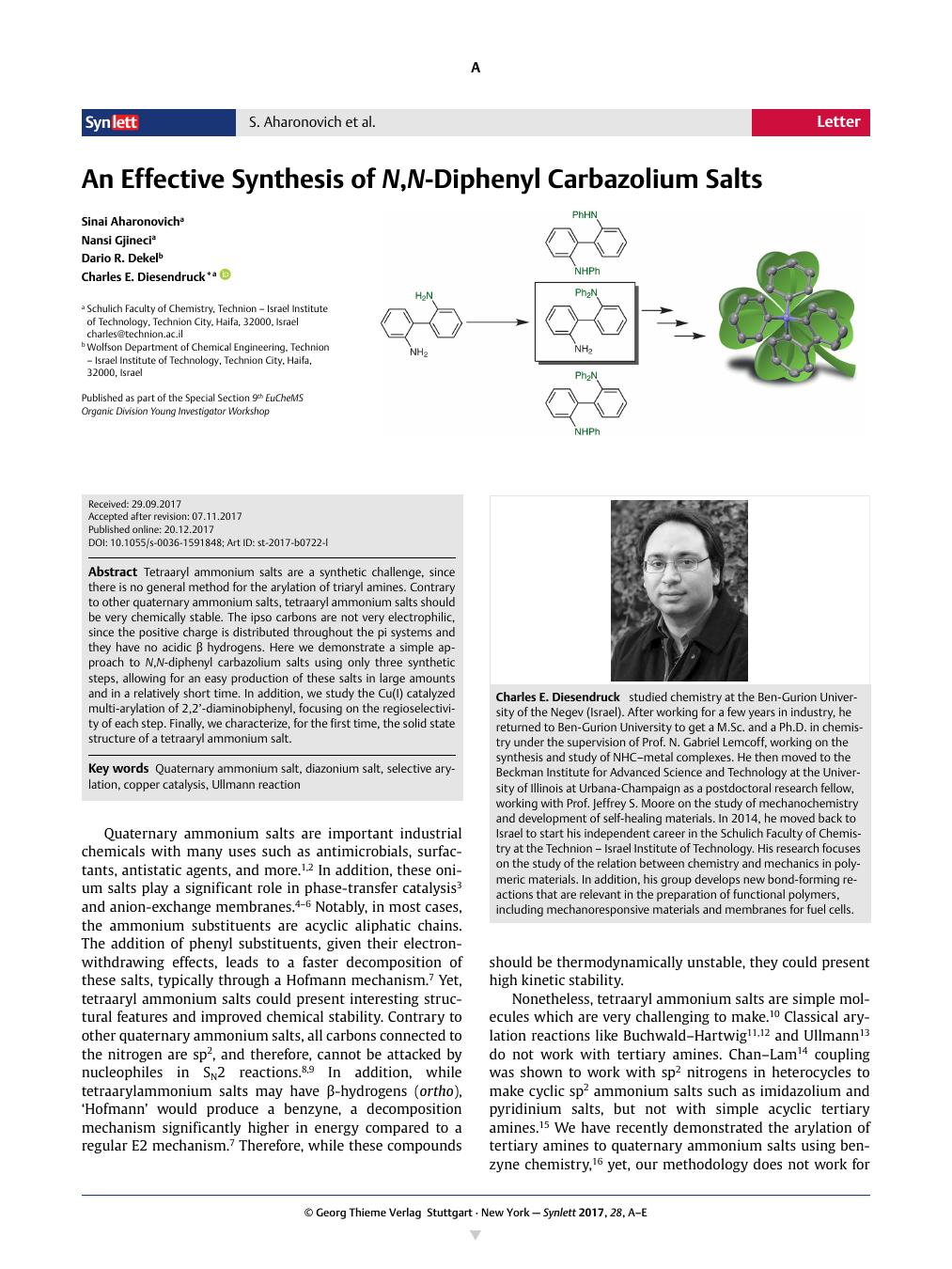
Tetraaryl ammonium salts are a synthetic challenge, since there is no general method for the arylation of triaryl amines. Contrary to other quaternary ammonium salts, tetraaryl ammonium salts should be very chemically stable. The ipso carbons are not very electrophilic, since the positive charge is distributed throughout the pi systems and they have no acidic β hydrogens. Here we demonstrate a simple approach to N,N-diphenyl carbazolium salts using only three synthetic steps, allowing for an easy production of these salts in large amounts and in a relatively short time. In addition, we study the Cu(I) catalyzed multi-arylation of 2,2’-diaminobiphenyl, focusing on the regioselectivity of each step. Finally, we characterize, for the first time, the solid state structure of a tetraaryl ammonium salt.
中文翻译:

N,N-二苯基咔唑盐的有效合成
四芳基铵盐是一个合成挑战,因为没有用于三芳基胺芳基化的通用方法。与其他季铵盐相反,四芳基铵盐的化学性质应该非常稳定。ipso 碳不是很亲电,因为正电荷分布在整个 pi 系统中,并且它们没有酸性 β 氢。在这里,我们展示了一种仅使用三个合成步骤的 N,N-二苯基咔唑盐的简单方法,可以在相对较短的时间内轻松地大量生产这些盐。此外,我们研究了 Cu(I) 催化的 2,2'-二氨基联苯的多芳基化,重点是每个步骤的区域选择性。最后,我们首次表征了四芳基铵盐的固态结构。
更新日期:2017-12-20
中文翻译:

N,N-二苯基咔唑盐的有效合成
四芳基铵盐是一个合成挑战,因为没有用于三芳基胺芳基化的通用方法。与其他季铵盐相反,四芳基铵盐的化学性质应该非常稳定。ipso 碳不是很亲电,因为正电荷分布在整个 pi 系统中,并且它们没有酸性 β 氢。在这里,我们展示了一种仅使用三个合成步骤的 N,N-二苯基咔唑盐的简单方法,可以在相对较短的时间内轻松地大量生产这些盐。此外,我们研究了 Cu(I) 催化的 2,2'-二氨基联苯的多芳基化,重点是每个步骤的区域选择性。最后,我们首次表征了四芳基铵盐的固态结构。



























 京公网安备 11010802027423号
京公网安备 11010802027423号