Acta Biomaterialia ( IF 9.7 ) Pub Date : 2017-12-20 , DOI: 10.1016/j.actbio.2017.12.010 Liangliang Huang , Lei Zhu , Xiaowei Shi , Bing Xia , Zhongyang Liu , Shu Zhu , Yafeng Yang , Teng Ma , Pengzhen Cheng , Kai Luo , Jinghui Huang , Zhuojing Luo
|
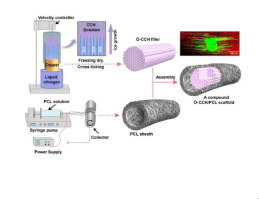
Scaffolds with inner fillers that convey directional guidance cues represent promising candidates for nerve repair. However, incorrect positioning or non-uniform distribution of intraluminal fillers might result in regeneration failure. In addition, proper porosity (to enhance nutrient and oxygen exchange but prevent fibroblast infiltration) and mechanical properties (to ensure fixation and to protect regenerating axons from compression) of the outer sheath are also highly important for constructing advanced nerve scaffolds. In this study, we constructed a compound scaffold using a stage-wise strategy, including directionally freezing orientated collagen-chitosan (O-CCH) filler, electrospinning poly(ε-caprolactone) (PCL) sheaths and assembling O-CCH/PCL scaffolds. Based on scanning electron microscopy (SEM) and mechanical tests, a blend of collagen/chitosan (1:1) was selected for filler fabrication, and a wall thickness of 400 μm was selected for PCL sheath production. SEM and three-dimensional (3D) reconstruction further revealed that the O-CCH filler exhibited a uniform, longitudinally oriented microstructure (over 85% of pores were 20-50 μm in diameter). The electrospun PCL porous sheath with pore sizes of 6.5 ± 3.3 μm prevented fibroblast invasion. The PCL sheath exhibited comparable mechanical properties to commercially available nerve conduits, and the O-CCH filler showed a physiologically relevant substrate stiffness of 2.0 ± 0.4 kPa. The differential degradation time of the filler and sheath allows the O-CCH/PCL scaffold to protect regenerating axons from compression stress while providing enough space for regenerating nerves. In vitro and in vivo studies indicated that the O-CCH/PCL scaffolds could promote axonal regeneration and Schwann cell migration. More importantly, functional results indicated that the CCH/PCL compound scaffold induced comparable functional recovery to that of the autograft group at the end of the study. Our findings demonstrated that the O-CCH/PCL scaffold with uniform longitudinal guidance filler and a porous sheath exhibits favorable properties for clinical use and promotes nerve regeneration and functional recovery. The O-CCH/PCL scaffold provides a promising new path for developing an optimal therapeutic alternative for peripheral nerve reconstruction.
Statement of Significance
Scaffolds with inner fillers displaying directional guidance cues represent a promising candidate for nerve repair. However, further clinical translation should pay attention to the problem of non-uniform distribution of inner fillers, the porosity and mechanical properties of the outer sheath and the morphological design facilitating operation. In this study, a stage-wise fabrication strategy was used, which made it possible to develop an O-CCH/PCL compound scaffold with a uniform longitudinally oriented inner filler and a porous outer sheath. The uniform distribution of the pores in the O-CCH/PCL scaffold provides a solution to resolve the problem of non-uniform distribution of inner fillers, which impede the clinical translation of scaffolds with longitudinal microstructured fillers, especially for aligned-fiber-based scaffolds. In vitro and in vivo studies indicated that the O-CCH/PCL scaffolds could provide topographical cues for axonal regeneration and SC migration, which were not found for random scaffolds (with random microstructure resemble sponge-based scaffolds). The electrospun porous PCL sheath of the O-CCH/PCL scaffold not only prevented fibroblast infiltration, but also satisfied the mechanical requirements for clinical use, paving the way for clinical translation. The differential degradation time of the O-CCH filler and the PCL sheath makes O-CCH/PCL scaffold able to provide long protection for regenerating axons from compression stress, but enough space for regenerating nerve. These findings highlight the possibility of developing an optimal therapeutic alternative for nerve defects using the O-CCH/PCL scaffold.
中文翻译:

具有均匀的纵向导向提示和多孔鞘的复合支架可促进体内周围神经的再生
具有内部填充物的支架可以传达方向性的指导信息,是神经修复的有希望的候选者。但是,管腔内填充物的不正确定位或不均匀分布可能会导致再生失败。另外,外鞘的适当孔隙率(以增强营养和氧交换,但防止成纤维细胞浸润)和机械性能(以确保固定并保护再生轴突不受压迫)对于构建高级神经支架也非常重要。在这项研究中,我们使用分阶段策略构建了复合支架,包括定向冷冻定向的胶原壳聚糖(O-CCH)填料,静电纺丝聚(ε-己内酯)(PCL)护套和组装O-CCH / PCL支架。基于扫描电子显微镜(SEM)和机械测试,选择胶原蛋白/壳聚糖(1:1)的混合物进行填充料制造,选择壁厚为400μm的PCL护套。SEM和三维(3D)重建进一步表明,O-CCH填料表现出均匀的纵向取向的微观结构(超过85%的孔的直径为20-50μm)。电纺PCL多孔护套的孔径为6.5±3.3μm,可防止成纤维细胞浸润。PCL鞘表现出可与市售神经导管相媲美的机械性能,而O-CCH填料表现出2.0±0.4 kPa的生理相关底物硬度。填充物和鞘的不同降解时间允许O-CCH / PCL支架保护再生轴突免受压缩应力,同时为再生神经提供足够的空间。选择1)进行填充料制造,选择壁厚400μm进行PCL护套生产。SEM和三维(3D)重建进一步表明,O-CCH填料表现出均匀的纵向取向的微观结构(超过85%的孔的直径为20-50μm)。电纺PCL多孔护套的孔径为6.5±3.3μm,可防止成纤维细胞浸润。PCL鞘表现出可与市售神经导管相媲美的机械性能,而O-CCH填料表现出2.0±0.4 kPa的生理相关底物硬度。填充物和鞘的不同降解时间允许O-CCH / PCL支架保护再生轴突免受压缩应力,同时为再生神经提供足够的空间。选择1)进行填充料制造,选择壁厚400μm进行PCL护套生产。SEM和三维(3D)重建进一步表明,O-CCH填料表现出均匀的纵向取向的微观结构(超过85%的孔的直径为20-50μm)。电纺PCL多孔护套的孔径为6.5±3.3μm,可防止成纤维细胞浸润。PCL鞘表现出可与市售神经导管相媲美的机械性能,而O-CCH填料表现出2.0±0.4 kPa的生理相关底物硬度。填充物和鞘的不同降解时间允许O-CCH / PCL支架保护再生轴突免受压缩应力,同时为再生神经提供足够的空间。选择PCL护套的壁厚为400μm。SEM和三维(3D)重建进一步表明,O-CCH填料表现出均匀的纵向取向的微观结构(超过85%的孔的直径为20-50μm)。电纺PCL多孔护套的孔径为6.5±3.3μm,可防止成纤维细胞浸润。PCL鞘表现出可与市售神经导管相媲美的机械性能,而O-CCH填料表现出2.0±0.4 kPa的生理相关底物硬度。填充物和鞘的不同降解时间允许O-CCH / PCL支架保护再生轴突免受压缩应力,同时为再生神经提供足够的空间。选择PCL护套的壁厚为400μm。SEM和三维(3D)重建进一步表明,O-CCH填料表现出均匀的纵向取向的微观结构(超过85%的孔的直径为20-50μm)。电纺PCL多孔护套的孔径为6.5±3.3μm,可防止成纤维细胞浸润。PCL鞘表现出可与市售神经导管相媲美的机械性能,而O-CCH填料表现出2.0±0.4 kPa的生理相关底物硬度。填充物和鞘的不同降解时间允许O-CCH / PCL支架保护再生轴突免受压缩应力,同时为再生神经提供足够的空间。SEM和三维(3D)重建进一步表明,O-CCH填料表现出均匀的纵向取向的微观结构(超过85%的孔的直径为20-50μm)。电纺PCL多孔护套的孔径为6.5±3.3μm,可防止成纤维细胞浸润。PCL鞘表现出可与市售神经导管相媲美的机械性能,而O-CCH填料表现出2.0±0.4 kPa的生理相关底物硬度。填充物和鞘的不同降解时间允许O-CCH / PCL支架保护再生轴突免受压缩应力,同时为再生神经提供足够的空间。SEM和三维(3D)重建进一步表明,O-CCH填料表现出均匀的纵向取向的微观结构(超过85%的孔的直径为20-50μm)。电纺PCL多孔护套的孔径为6.5±3.3μm,可防止成纤维细胞浸润。PCL鞘表现出可与市售神经导管相媲美的机械性能,而O-CCH填料表现出2.0±0.4 kPa的生理相关底物硬度。填充物和鞘的不同降解时间允许O-CCH / PCL支架保护再生轴突免受压缩应力,同时为再生神经提供足够的空间。3μm可防止成纤维细胞浸润。PCL鞘表现出可与市售神经导管相媲美的机械性能,而O-CCH填料表现出2.0±0.4 kPa的生理相关底物硬度。填充物和鞘的不同降解时间允许O-CCH / PCL支架保护再生轴突免受压缩应力,同时为再生神经提供足够的空间。3μm可防止成纤维细胞浸润。PCL鞘表现出可与市售神经导管相媲美的机械性能,而O-CCH填料表现出2.0±0.4 kPa的生理相关底物硬度。填充物和鞘的不同降解时间允许O-CCH / PCL支架保护再生轴突免受压缩应力,同时为再生神经提供足够的空间。体外和体内研究表明,O-CCH / PCL支架可以促进轴突再生和雪旺细胞迁移。更重要的是,功能结果表明,在研究结束时,CCH / PCL复合支架诱导的功能恢复与自体移植组相当。我们的研究结果表明,具有均匀的纵向引导填充物和多孔鞘的O-CCH / PCL支架在临床上具有良好的性能,并能促进神经再生和功能恢复。O-CCH / PCL支架为开发用于周围神经重建的最佳治疗替代方法提供了有希望的新途径。
重要声明
具有内部填充物的支架显示方向性指导提示,是神经修复的有前途的候选者。但是,进一步的临床翻译应注意内填充物分布不均匀,外鞘的孔隙率和机械性能以及便于操作的形态学设计等问题。在这项研究中,使用了分阶段的制造策略,这使得开发具有均匀纵向取向的内部填充物和多孔外部护套的O-CCH / PCL复合支架成为可能。O-CCH / PCL支架中孔的均匀分布为解决内部填充物分布不均匀的问题提供了解决方案,该问题阻碍了具有纵向微结构填充物的支架的临床翻译,特别是对于基于纤维的支架。体外和体内研究表明,O-CCH / PCL支架可以为轴突再生和SC迁移提供地形线索,而随机支架(其微观结构类似于海绵支架)则没有发现。O-CCH / PCL支架的电纺多孔PCL护套不仅防止了成纤维细胞浸润,而且满足了临床使用的机械要求,为临床翻译铺平了道路。O-CCH填充物和PCL护套的降解时间不同,使得O-CCH / PCL支架能够为轴突免受压迫应力提供长久的保护,但为神经再生提供了足够的空间。这些发现凸显了使用O-CCH / PCL支架开发神经缺陷的最佳治疗替代方案的可能性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号