当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoinduced Rearrangement of Dienones and Santonin Rerouted by Amines
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-12-20 , DOI: 10.1002/anie.201710463 Zhipeng Zhang 1 , Maxim Ratnikov 1 , Glen Spraggon 1 , Phil B. Alper 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2017-12-20 , DOI: 10.1002/anie.201710463 Zhipeng Zhang 1 , Maxim Ratnikov 1 , Glen Spraggon 1 , Phil B. Alper 1
Affiliation
|
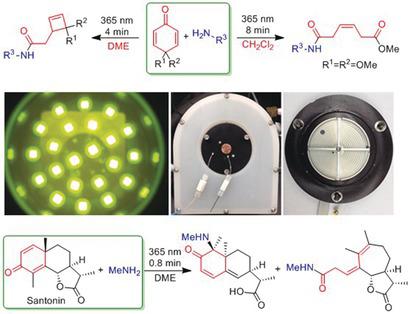
The photoinduced rearrangement pathways of simple 2,5‐dienones and the natural product santonin were found to be effectively rerouted by amines, giving rise to unprecedented products. Either cis olefins or cyclobutenes were obtained from 4,4‐disubstituted 2,5‐dienone upon irradiation (365 nm) in the presence of various amines depending on the solvent. Previously undescribed [4.4.0] and [5.3.0] fused‐ring‐containing products were obtained when santonin was irradiated (365 nm) in the presence of methylamine. The amines present in these reactions were incorporated into the products by means of amide‐group formation.
中文翻译:

胺重排二烯酮和桑托宁的光诱导重排
发现简单的2,5-二壬烯和天然产物桑通宁的光诱导重排途径可以被胺有效地改变路线,从而产生了前所未有的产物。或者顺式从得到的烯烃或环丁烯当在取决于溶剂各种胺的存在下照射(365纳米)4,4-二取代的-2,5-二烯酮。当在甲胺存在下照射桑顿宁(365 nm)时,获得了以前未描述的[4.4.0]和[5.3.0]稠环产品。这些反应中存在的胺通过酰胺基的形成被掺入产物中。
更新日期:2017-12-20
中文翻译:

胺重排二烯酮和桑托宁的光诱导重排
发现简单的2,5-二壬烯和天然产物桑通宁的光诱导重排途径可以被胺有效地改变路线,从而产生了前所未有的产物。或者顺式从得到的烯烃或环丁烯当在取决于溶剂各种胺的存在下照射(365纳米)4,4-二取代的-2,5-二烯酮。当在甲胺存在下照射桑顿宁(365 nm)时,获得了以前未描述的[4.4.0]和[5.3.0]稠环产品。这些反应中存在的胺通过酰胺基的形成被掺入产物中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号