当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effects of Chloride Ions on Dissolution, ROS Generation, and Toxicity of Silver Nanoparticles under UV Irradiation
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.est.7b04547 Yang Li 1 , Jian Zhao 1 , Enxiang Shang 1 , Xinghui Xia 1 , Junfeng Niu 2 , John Crittenden 3
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.est.7b04547 Yang Li 1 , Jian Zhao 1 , Enxiang Shang 1 , Xinghui Xia 1 , Junfeng Niu 2 , John Crittenden 3
Affiliation
|
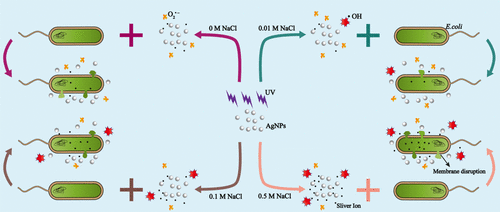
This work investigates the effect of chloride ion (Cl–) on dissolution, reactive oxygen species (ROS) generation, and toxicity of citrate-coated silver nanoparticles (AgNPs) under UV irradiation. The dissolution rate was decreased by 0.01 M Cl– due to AgCl passivation on the AgNP surface. By contrast, high concentrations of Cl– (0.1 or 0.5 M) promoted dissolution due to the formation of soluble Ag–Cl complexes (AgClx1–x). The generation of O2•– in the AgNPs/Cl–/UV system was promoted by 0.01 M Cl–, whereas it was retarded by 0.1 or 0.5 M Cl–, which was probably because the aggregation of AgNPs at high ionic strength reduced the nanoparticles’ surface areas for radical formation. Additionally, Cl– contributed to •OH generation in the AgNPs/Cl–/UV system, in which the produced •OH concentrations increased with increasing Cl– concentrations. The reduction reaction between silver ions and O2•– resulted in lower dissolution rates of AgNPs/Cl– mixtures under UV irradiation than those in the dark. The phototoxicity of AgNPs toward E. coli with different concentrations of Cl– followed the order of 0.5 M > 0 M > 0.1 M > 0.01 M. Both ROS and dissolved Ag played significant role in the phototoxicity of AgNPs. This work demonstrates the potential importance of anions in the fate and biological impact of AgNPs.
中文翻译:

氯离子对紫外线辐照下银纳米粒子的溶解,ROS生成和毒性的影响
这项工作调查氯离子的作用(CL - )对溶出,活性氧(ROS)的产生,和柠檬酸盐涂覆的银纳米颗粒(的AgNPs)毒性UV辐射下。由于AgNP表面上的AgCl钝化,溶解速率降低了0.01 M Cl –。相比之下,高浓度的Cl –(0.1或0.5 M)由于可溶性Ag–Cl络合物(AgCl x 1– x)的形成而促进了溶解。0.01 M Cl –促进了AgNPs / Cl – / UV系统中O 2 •–的生成,而0.1或0.5 M Cl –则阻止了O 2 •–的生成。,这可能是因为高离子强度的AgNP的聚集减少了纳米颗粒形成自由基的表面积。此外,氯-促成•OH产生在的AgNPs /氯- / UV系统,其中所产生的•OH浓度随增加氯-浓度。银离子和O 2 •–之间的还原反应导致紫外线下的AgNPs / Cl –混合物的溶解速率低于黑暗中的AgNPs / Cl –混合物的溶解速率。AgNPs对不同浓度Cl –的大肠杆菌的光毒性遵循0.5 M> 0 M> 0.1 M> 0.01 M的顺序。ROS和溶解的Ag在AgNPs的光毒性中均起着重要作用。这项工作证明了阴离子对AgNPs的命运和生物学影响的潜在重要性。
更新日期:2018-01-03
中文翻译:

氯离子对紫外线辐照下银纳米粒子的溶解,ROS生成和毒性的影响
这项工作调查氯离子的作用(CL - )对溶出,活性氧(ROS)的产生,和柠檬酸盐涂覆的银纳米颗粒(的AgNPs)毒性UV辐射下。由于AgNP表面上的AgCl钝化,溶解速率降低了0.01 M Cl –。相比之下,高浓度的Cl –(0.1或0.5 M)由于可溶性Ag–Cl络合物(AgCl x 1– x)的形成而促进了溶解。0.01 M Cl –促进了AgNPs / Cl – / UV系统中O 2 •–的生成,而0.1或0.5 M Cl –则阻止了O 2 •–的生成。,这可能是因为高离子强度的AgNP的聚集减少了纳米颗粒形成自由基的表面积。此外,氯-促成•OH产生在的AgNPs /氯- / UV系统,其中所产生的•OH浓度随增加氯-浓度。银离子和O 2 •–之间的还原反应导致紫外线下的AgNPs / Cl –混合物的溶解速率低于黑暗中的AgNPs / Cl –混合物的溶解速率。AgNPs对不同浓度Cl –的大肠杆菌的光毒性遵循0.5 M> 0 M> 0.1 M> 0.01 M的顺序。ROS和溶解的Ag在AgNPs的光毒性中均起着重要作用。这项工作证明了阴离子对AgNPs的命运和生物学影响的潜在重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号