当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Methodology for the Validation of Isotopic Analyses by Mass Spectrometry in Stable-Isotope Labeling Experiments
Analytical Chemistry ( IF 7.4 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.analchem.7b03886 Maud Heuillet 1, 2 , Floriant Bellvert 1, 2 , Edern Cahoreau 1, 2 , Fabien Letisse 1, 3 , Pierre Millard 1 , Jean-Charles Portais 1, 2, 3
Analytical Chemistry ( IF 7.4 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.analchem.7b03886 Maud Heuillet 1, 2 , Floriant Bellvert 1, 2 , Edern Cahoreau 1, 2 , Fabien Letisse 1, 3 , Pierre Millard 1 , Jean-Charles Portais 1, 2, 3
Affiliation
|
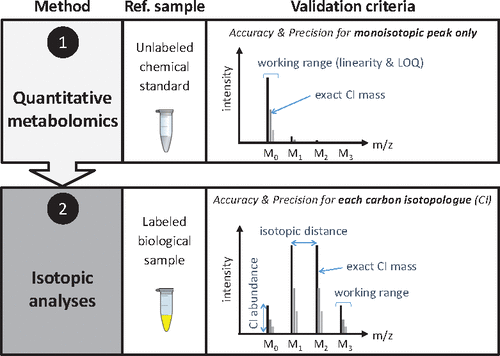
Stable-isotope labeling experiments (ILEs) are widely used to investigate the topology and operation of metabolic networks. The quality of isotopic data collected in ILEs is of utmost importance to ensure reliable biological interpretations, but current evaluation approaches are limited due to a lack of suitable reference material and relevant evaluation criteria. In this work, we present a complete methodology to evaluate mass spectrometry (MS) methods used for quantitative isotopic studies of metabolic systems. This methodology, based on a biological sample containing metabolites with controlled labeling patterns, exploits different quality metrics specific to isotopic analyses (accuracy and precision of isotopologue masses, abundances, and mass shifts and isotopic working range). We applied this methodology to evaluate a novel LC-MS method for the analysis of amino acids, which was tested on high resolution (Orbitrap operating in full scan mode) and low resolution (triple quadrupole operating in multiple reaction monitoring mode) mass spectrometers. Results show excellent accuracy and precision over a large working range and revealed matrix-specific as well as mode-specific characteristics. The proposed methodology can identify reliable (and unreliable) isotopic data in an easy and straightforward way and efficiently supports the identification of sources of systematic biases as well as of the main factors that influence the overall accuracy and precision of measurements. This approach is generic and can be used to validate isotopic analyses on different matrices, analytical platforms, labeled elements, or classes of metabolites. It is expected to strengthen the reliability of isotopic measurements and thereby the biological value of ILEs.
中文翻译:

质谱在稳定同位素标记实验中验证同位素分析的方法学
稳定同位素标记实验(ILE)被广泛用于研究代谢网络的拓扑和操作。在ILE中收集同位素数据的质量对于确保可靠的生物学解释至关重要,但是由于缺乏合适的参考材料和相关的评估标准,目前的评估方法受到限制。在这项工作中,我们提出了一种用于评估代谢系统定量同位素研究的质谱(MS)方法的完整方法。该方法基于含有代谢物的生物样品,该代谢物具有可控的标记模式,利用了同位素分析所特有的不同质量指标(同位素同位素质量,丰度,质量偏移和同位素工作范围的准确性和精密度)。我们应用此方法评估了一种用于氨基酸分析的新型LC-MS方法,该方法已在高分辨率(Orbitrap在全扫描模式下运行)和低分辨率(三重四极在多反应监测模式下运行)质谱仪上进行了测试。结果表明,在较大的工作范围内,其准确性和精密度均很高,并显示出特定于矩阵和特定于模式的特性。所提出的方法可以以简单,直接的方式识别可靠(和不可靠)的同位素数据,并有效地支持识别系统偏差的来源以及影响总体测量精度和精确度的主要因素。此方法是通用方法,可用于验证不同基质,分析平台,标记元素,或代谢物类别。有望增强同位素测量的可靠性,从而增强ILE的生物学价值。
更新日期:2018-01-17
中文翻译:

质谱在稳定同位素标记实验中验证同位素分析的方法学
稳定同位素标记实验(ILE)被广泛用于研究代谢网络的拓扑和操作。在ILE中收集同位素数据的质量对于确保可靠的生物学解释至关重要,但是由于缺乏合适的参考材料和相关的评估标准,目前的评估方法受到限制。在这项工作中,我们提出了一种用于评估代谢系统定量同位素研究的质谱(MS)方法的完整方法。该方法基于含有代谢物的生物样品,该代谢物具有可控的标记模式,利用了同位素分析所特有的不同质量指标(同位素同位素质量,丰度,质量偏移和同位素工作范围的准确性和精密度)。我们应用此方法评估了一种用于氨基酸分析的新型LC-MS方法,该方法已在高分辨率(Orbitrap在全扫描模式下运行)和低分辨率(三重四极在多反应监测模式下运行)质谱仪上进行了测试。结果表明,在较大的工作范围内,其准确性和精密度均很高,并显示出特定于矩阵和特定于模式的特性。所提出的方法可以以简单,直接的方式识别可靠(和不可靠)的同位素数据,并有效地支持识别系统偏差的来源以及影响总体测量精度和精确度的主要因素。此方法是通用方法,可用于验证不同基质,分析平台,标记元素,或代谢物类别。有望增强同位素测量的可靠性,从而增强ILE的生物学价值。



























 京公网安备 11010802027423号
京公网安备 11010802027423号