Immunity ( IF 32.4 ) Pub Date : 2017-12-19 , DOI: 10.1016/j.immuni.2017.11.024 Jil Sander , Susanne V. Schmidt , Branko Cirovic , Naomi McGovern , Olympia Papantonopoulou , Anna-Lena Hardt , Anna C. Aschenbrenner , Christoph Kreer , Thomas Quast , Alexander M. Xu , Lisa M. Schmidleithner , Heidi Theis , Lan Do Thi Huong , Hermi Rizal Bin Sumatoh , Mario A.R. Lauterbach , Jonas Schulte-Schrepping , Patrick Günther , Jia Xue , Kevin Baßler , Thomas Ulas , Kathrin Klee , Natalie Katzmarski , Stefanie Herresthal , Wolfgang Krebs , Bianca Martin , Eicke Latz , Kristian Händler , Michael Kraut , Waldemar Kolanus , Marc Beyer , Christine S. Falk , Bettina Wiegmann , Sven Burgdorf , Nicholas A. Melosh , Evan W. Newell , Florent Ginhoux , Andreas Schlitzer , Joachim L. Schultze
|
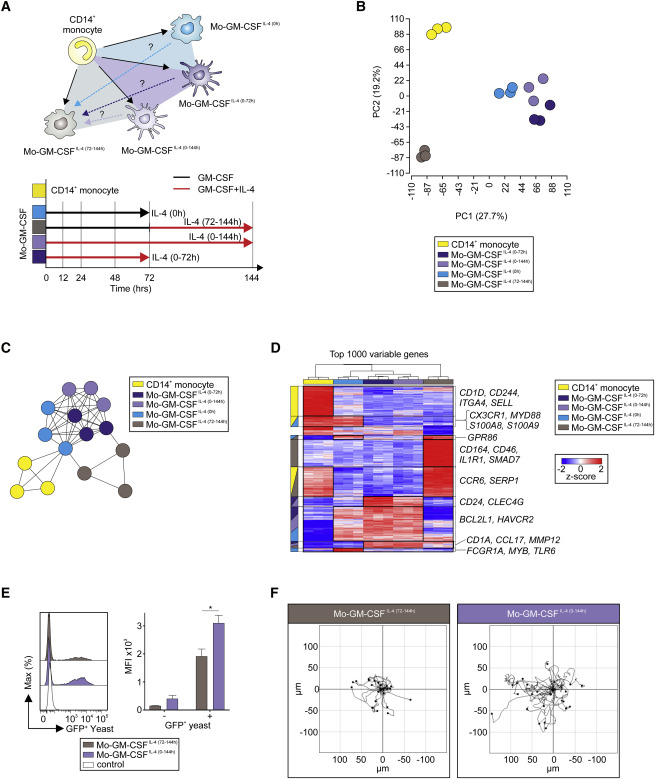
Human in vitro generated monocyte-derived dendritic cells (moDCs) and macrophages are used clinically, e.g., to induce immunity against cancer. However, their physiological counterparts, ontogeny, transcriptional regulation, and heterogeneity remains largely unknown, hampering their clinical use. High-dimensional techniques were used to elucidate transcriptional, phenotypic, and functional differences between human in vivo and in vitro generated mononuclear phagocytes to facilitate their full potential in the clinic. We demonstrate that monocytes differentiated by macrophage colony-stimulating factor (M-CSF) or granulocyte macrophage colony-stimulating factor (GM-CSF) resembled in vivo inflammatory macrophages, while moDCs resembled in vivo inflammatory DCs. Moreover, differentiated monocytes presented with profound transcriptomic, phenotypic, and functional differences. Monocytes integrated GM-CSF and IL-4 stimulation combinatorically and temporally, resulting in a mode- and time-dependent differentiation relying on NCOR2. Finally, moDCs are phenotypically heterogeneous and therefore necessitate the use of high-dimensional phenotyping to open new possibilities for better clinical tailoring of these cellular therapies.
中文翻译:

人类单核细胞的细胞分化受时间依赖性白介素4信号和转录调节因子NCOR2的调节。
人体外产生的单核细胞衍生的树突状细胞(moDC)和巨噬细胞可用于临床,例如,诱导抗癌免疫力。然而,它们的生理对应物,个体发育,转录调控和异质性仍然是未知的,从而妨碍了它们的临床应用。高维技术用于阐明人体内和体外产生的单核吞噬细胞之间的转录,表型和功能差异,以促进其在临床中的全部潜力。我们证明,由巨噬细胞集落刺激因子(M-CSF)或粒细胞巨噬细胞集落刺激因子(GM-CSF)分化的单核细胞类似于体内炎性巨噬细胞,而moDC类似于体内炎症性DC。此外,分化的单核细胞呈现出深刻的转录组,表型和功能差异。单核细胞组合性和暂时性地整合了GM-CSF和IL-4刺激,导致依赖NCOR2的模式和时间依赖性分化。最后,moDC在表型上是异质的,因此有必要使用高维表型来为这些细胞疗法的更好的临床定制开辟新的可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号