Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma
JAMA ( IF 120.7 ) Pub Date : 2017-12-19 , DOI: 10.1001/jama.2017.18718 Roger Stupp 1, 2, 3 , Sophie Taillibert 4 , Andrew Kanner 5 , William Read 6, 7 , David M. Steinberg 8 , Benoit Lhermitte 2 , Steven Toms 9 , Ahmed Idbaih 4 , Manmeet S. Ahluwalia 10 , Karen Fink 11 , Francesco Di Meco 12 , Frank Lieberman 13 , Jay-Jiguang Zhu 14, 15 , Giuseppe Stragliotto 16 , David D. Tran 17 , Steven Brem 18, 19 , Andreas F. Hottinger 2 , Eilon D. Kirson 20 , Gitit Lavy-Shahaf 20 , Uri Weinberg 20 , Chae-Yong Kim 21 , Sun-Ha Paek 22 , Garth Nicholas 23 , Jordi Bruna 24 , Hal Hirte 25 , Michael Weller 3 , Yoram Palti 20 , Monika E. Hegi 2 , Zvi Ram 5
JAMA ( IF 120.7 ) Pub Date : 2017-12-19 , DOI: 10.1001/jama.2017.18718 Roger Stupp 1, 2, 3 , Sophie Taillibert 4 , Andrew Kanner 5 , William Read 6, 7 , David M. Steinberg 8 , Benoit Lhermitte 2 , Steven Toms 9 , Ahmed Idbaih 4 , Manmeet S. Ahluwalia 10 , Karen Fink 11 , Francesco Di Meco 12 , Frank Lieberman 13 , Jay-Jiguang Zhu 14, 15 , Giuseppe Stragliotto 16 , David D. Tran 17 , Steven Brem 18, 19 , Andreas F. Hottinger 2 , Eilon D. Kirson 20 , Gitit Lavy-Shahaf 20 , Uri Weinberg 20 , Chae-Yong Kim 21 , Sun-Ha Paek 22 , Garth Nicholas 23 , Jordi Bruna 24 , Hal Hirte 25 , Michael Weller 3 , Yoram Palti 20 , Monika E. Hegi 2 , Zvi Ram 5
Affiliation
|
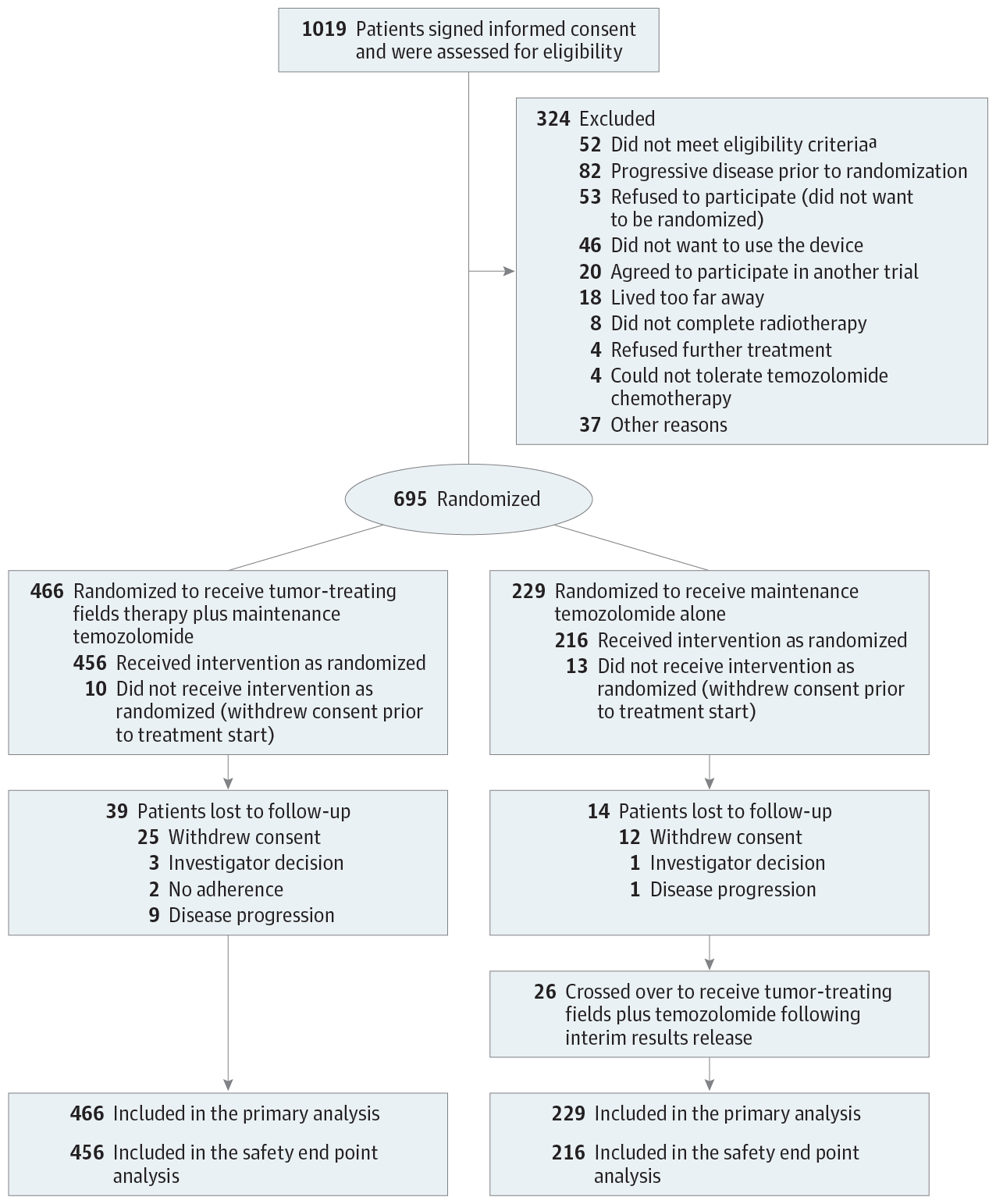
Importance Tumor-treating fields (TTFields) is an antimitotic treatment modality that interferes with glioblastoma cell division and organelle assembly by delivering low-intensity alternating electric fields to the tumor. Objective To investigate whether TTFields improves progression-free and overall survival of patients with glioblastoma, a fatal disease that commonly recurs at the initial tumor site or in the central nervous system. Design, Setting, and Participants In this randomized, open-label trial, 695 patients with glioblastoma whose tumor was resected or biopsied and had completed concomitant radiochemotherapy (median time from diagnosis to randomization, 3.8 months) were enrolled at 83 centers (July 2009-2014) and followed up through December 2016. A preliminary report from this trial was published in 2015; this report describes the final analysis. Interventions Patients were randomized 2:1 to TTFields plus maintenance temozolomide chemotherapy (n = 466) or temozolomide alone (n = 229). The TTFields, consisting of low-intensity, 200 kHz frequency, alternating electric fields, was delivered (≥ 18 hours/d) via 4 transducer arrays on the shaved scalp and connected to a portable device. Temozolomide was administered to both groups (150-200 mg/m2) for 5 days per 28-day cycle (6-12 cycles). Main Outcomes and Measures Progression-free survival (tested at &agr; = .046). The secondary end point was overall survival (tested hierarchically at &agr; = .048). Analyses were performed for the intent-to-treat population. Adverse events were compared by group. Results Of the 695 randomized patients (median age, 56 years; IQR, 48-63; 473 men [68%]), 637 (92%) completed the trial. Median progression-free survival from randomization was 6.7 months in the TTFields-temozolomide group and 4.0 months in the temozolomide-alone group (HR, 0.63; 95% CI, 0.52-0.76; P < .001). Median overall survival was 20.9 months in the TTFields-temozolomide group vs 16.0 months in the temozolomide-alone group (HR, 0.63; 95% CI, 0.53-0.76; P < .001). Systemic adverse event frequency was 48% in the TTFields-temozolomide group and 44% in the temozolomide-alone group. Mild to moderate skin toxicity underneath the transducer arrays occurred in 52% of patients who received TTFields-temozolomide vs no patients who received temozolomide alone. Conclusions and Relevance In the final analysis of this randomized clinical trial of patients with glioblastoma who had received standard radiochemotherapy, the addition of TTFields to maintenance temozolomide chemotherapy vs maintenance temozolomide alone, resulted in statistically significant improvement in progression-free survival and overall survival. These results are consistent with the previous interim analysis. Trial Registration clinicaltrials.gov Identifier: NCT00916409
中文翻译:

肿瘤治疗野加维持替莫唑胺与单独维持替莫唑胺对胶质母细胞瘤患者生存率的影响
重要性肿瘤治疗场 (TTFields) 是一种抗有丝分裂治疗方式,通过向肿瘤提供低强度交变电场来干扰胶质母细胞瘤细胞分裂和细胞器组装。目的 研究 TTFields 是否能改善胶质母细胞瘤患者的无进展生存期和总生存期,胶质母细胞瘤是一种通常在初始肿瘤部位或中枢神经系统复发的致命疾病。设计、设置和参与者 在这项随机、开放标签试验中,在 83 个中心招募了 695 名胶质母细胞瘤患者,他们的肿瘤被切除或活检并完成了伴随放化疗(从诊断到随机化的中位时间,3.8 个月)(2009 年 7 月 - 2014)并随访至 2016 年 12 月。该试验的初步报告于 2015 年发表;本报告描述了最终分析。干预 患者按 2:1 随机接受 TTFields 加替莫唑胺维持化疗(n = 466)或单独接受替莫唑胺(n = 229)。TTFields 由低强度、200 kHz 频率的交变电场组成,通过剃光头皮上的 4 个换能器阵列传送(≥ 18 小时/天)并连接到便携式设备。替莫唑胺给予两组(150-200 mg/m2),每 28 天周期(6-12 个周期)共 5 天。主要结果和测量无进展生存期(在 &agr; = .046 时测试)。次要终点是总生存期(在 &agr; = .048 进行分层测试)。对意向治疗人群进行了分析。按组比较不良事件。结果 在 695 名随机患者中(中位年龄,56 岁;IQR,48-63;473 名男性 [68%]),637 (92%) 人完成了试验。TTFields-替莫唑胺组的中位无进展生存期为 6.7 个月,替莫唑胺单药组为 4.0 个月(HR,0.63;95% CI,0.52-0.76;P < .001)。TTFields-替莫唑胺组的中位总生存期为 20.9 个月,而替莫唑胺单药组为 16.0 个月(HR,0.63;95% CI,0.53-0.76;P < .001)。TTFields-替莫唑胺组的全身不良事件频率为 48%,替莫唑胺单药组为 44%。接受 TTFields-替莫唑胺治疗的患者中有 52% 的患者出现了换能器阵列下方的轻度至中度皮肤毒性,而单独接受替莫唑胺治疗的患者则没有。结论和相关性 在对接受标准放化疗的胶质母细胞瘤患者进行的这项随机临床试验的最终分析中,将 TTFields 添加到维持替莫唑胺化疗与单独维持替莫唑胺相比,导致无进展生存期和总生存期的统计学显着改善。这些结果与之前的中期分析一致。试验注册clinicaltrials.gov 标识符:NCT00916409
更新日期:2017-12-19
中文翻译:

肿瘤治疗野加维持替莫唑胺与单独维持替莫唑胺对胶质母细胞瘤患者生存率的影响
重要性肿瘤治疗场 (TTFields) 是一种抗有丝分裂治疗方式,通过向肿瘤提供低强度交变电场来干扰胶质母细胞瘤细胞分裂和细胞器组装。目的 研究 TTFields 是否能改善胶质母细胞瘤患者的无进展生存期和总生存期,胶质母细胞瘤是一种通常在初始肿瘤部位或中枢神经系统复发的致命疾病。设计、设置和参与者 在这项随机、开放标签试验中,在 83 个中心招募了 695 名胶质母细胞瘤患者,他们的肿瘤被切除或活检并完成了伴随放化疗(从诊断到随机化的中位时间,3.8 个月)(2009 年 7 月 - 2014)并随访至 2016 年 12 月。该试验的初步报告于 2015 年发表;本报告描述了最终分析。干预 患者按 2:1 随机接受 TTFields 加替莫唑胺维持化疗(n = 466)或单独接受替莫唑胺(n = 229)。TTFields 由低强度、200 kHz 频率的交变电场组成,通过剃光头皮上的 4 个换能器阵列传送(≥ 18 小时/天)并连接到便携式设备。替莫唑胺给予两组(150-200 mg/m2),每 28 天周期(6-12 个周期)共 5 天。主要结果和测量无进展生存期(在 &agr; = .046 时测试)。次要终点是总生存期(在 &agr; = .048 进行分层测试)。对意向治疗人群进行了分析。按组比较不良事件。结果 在 695 名随机患者中(中位年龄,56 岁;IQR,48-63;473 名男性 [68%]),637 (92%) 人完成了试验。TTFields-替莫唑胺组的中位无进展生存期为 6.7 个月,替莫唑胺单药组为 4.0 个月(HR,0.63;95% CI,0.52-0.76;P < .001)。TTFields-替莫唑胺组的中位总生存期为 20.9 个月,而替莫唑胺单药组为 16.0 个月(HR,0.63;95% CI,0.53-0.76;P < .001)。TTFields-替莫唑胺组的全身不良事件频率为 48%,替莫唑胺单药组为 44%。接受 TTFields-替莫唑胺治疗的患者中有 52% 的患者出现了换能器阵列下方的轻度至中度皮肤毒性,而单独接受替莫唑胺治疗的患者则没有。结论和相关性 在对接受标准放化疗的胶质母细胞瘤患者进行的这项随机临床试验的最终分析中,将 TTFields 添加到维持替莫唑胺化疗与单独维持替莫唑胺相比,导致无进展生存期和总生存期的统计学显着改善。这些结果与之前的中期分析一致。试验注册clinicaltrials.gov 标识符:NCT00916409



























 京公网安备 11010802027423号
京公网安备 11010802027423号