当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of the Cyclopiazonic Acid Family Using Sulfur Ylides.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-09 , DOI: 10.1002/anie.201712065 Oleksandr Zhurakovskyi 1 , Yunus E Türkmen 1 , Lorenz E Löffler 1 , Vijayalakshmi A Moorthie 1 , C Chun Chen 1 , Michael A Shaw 1 , Mark R Crimmin 1 , Marco Ferrara 1 , Mushtaq Ahmad 1 , Mehrnoosh Ostovar 1 , Johnathan V Matlock 1 , Varinder K Aggarwal 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-01-09 , DOI: 10.1002/anie.201712065 Oleksandr Zhurakovskyi 1 , Yunus E Türkmen 1 , Lorenz E Löffler 1 , Vijayalakshmi A Moorthie 1 , C Chun Chen 1 , Michael A Shaw 1 , Mark R Crimmin 1 , Marco Ferrara 1 , Mushtaq Ahmad 1 , Mehrnoosh Ostovar 1 , Johnathan V Matlock 1 , Varinder K Aggarwal 1
Affiliation
|
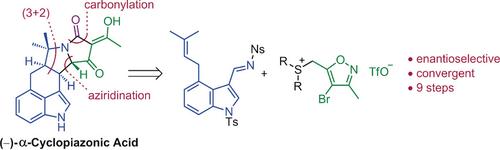
A convergent, nine-step (LLS), enantioselective synthesis of α-cyclopiazonic acid and related natural products is reported. The route features a) an enantioselective aziridination of an imine with a chiral sulfur ylide; b) a bioinspired (3+2)-cycloaddition of the aziridine onto an alkene; and c) installation of the acetyltetramic acid by an unprecedented tandem carbonylative lactamization/N-O cleavage of a bromoisoxazole.
中文翻译:

使用硫叶立德对映体合成环戊二酸家族。
据报道,α-环吡嗪酸及其相关天然产物的聚合,九步(LLS)对映选择性合成。该路线的特征是:a)亚胺与手性硫叶立德的对映选择性叠氮化;b)将氮丙啶生物启发(3 + 2)-环加成到烯烃上;c)通过空前的串联式羰基内酰胺化/溴异恶唑的NO裂解来安装乙酰基四酸。
更新日期:2018-01-09
中文翻译:

使用硫叶立德对映体合成环戊二酸家族。
据报道,α-环吡嗪酸及其相关天然产物的聚合,九步(LLS)对映选择性合成。该路线的特征是:a)亚胺与手性硫叶立德的对映选择性叠氮化;b)将氮丙啶生物启发(3 + 2)-环加成到烯烃上;c)通过空前的串联式羰基内酰胺化/溴异恶唑的NO裂解来安装乙酰基四酸。

























 京公网安备 11010802027423号
京公网安备 11010802027423号