当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Applied Potentials in Variable-Charge Reactive Force Fields for Electrochemical Systems
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.jpca.7b06064 Tao Liang , Andrew C. Antony 1 , Sneha A. Akhade 2 , Michael J. Janik , Susan B. Sinnott
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.jpca.7b06064 Tao Liang , Andrew C. Antony 1 , Sneha A. Akhade 2 , Michael J. Janik , Susan B. Sinnott
Affiliation
|
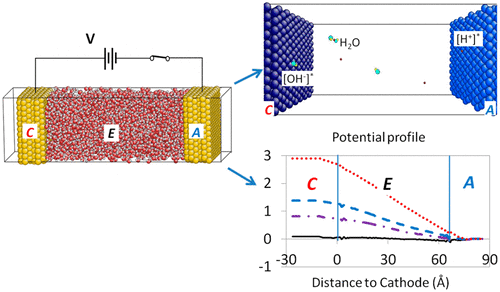
An atomic description of water dynamics and electrochemical properties at electrode–electrolyte interfaces is presented using molecular dynamics with the third generation of the charge-optimized many-body (COMB3) potential framework. Externally applied potentials in electrochemical applications were simulated by offsetting electronegativity on electrode atoms. This approach is incorporated into the variable charge scheme within COMB3 and is used to investigate electrochemical systems consisting of two Cu electrodes and a water electrolyte with varying concentrations of hydroxyls (OH–) and protons (H+). The interactions between the electronegativity offset method and the charge equilibration method in a variable charge scheme are analyzed. In addition, a charge equilibration method for electrochemical applications is proposed, where the externally applied potentials are treated by the electronegativity offset on the electrodes thus enforcing charge neutrality on the electrolyte. This method is able to qualitatively capture the relevant electrochemistry and predict consistently correct voltages with precalibration.
中文翻译:

电化学系统可变电荷反应力场中的应用电势
使用分子动力学和第三代电荷优化的多体(COMB3)势能框架,对电极-电解质界面的水动力学和电化学性质进行了原子描述。通过抵消电极原子上的电负性来模拟电化学应用中的外部施加电势。该方法被结合到内COMB3可变充电方案和用于研究由两个铜电极的电化学系统和具有变化的羟基(OH浓度水电解质- )和质子(H +)。分析了可变电荷方案中电负性偏移方法和电荷平衡方法之间的相互作用。另外,提出了一种用于电化学应用的电荷平衡方法,其中通过在电极上的电负性偏移来处理外部施加的电势,从而在电解质上施加电荷中性。这种方法能够定性地捕获相关的电化学,并通过预校准来预测一致的正确电压。
更新日期:2018-01-05
中文翻译:

电化学系统可变电荷反应力场中的应用电势
使用分子动力学和第三代电荷优化的多体(COMB3)势能框架,对电极-电解质界面的水动力学和电化学性质进行了原子描述。通过抵消电极原子上的电负性来模拟电化学应用中的外部施加电势。该方法被结合到内COMB3可变充电方案和用于研究由两个铜电极的电化学系统和具有变化的羟基(OH浓度水电解质- )和质子(H +)。分析了可变电荷方案中电负性偏移方法和电荷平衡方法之间的相互作用。另外,提出了一种用于电化学应用的电荷平衡方法,其中通过在电极上的电负性偏移来处理外部施加的电势,从而在电解质上施加电荷中性。这种方法能够定性地捕获相关的电化学,并通过预校准来预测一致的正确电压。



























 京公网安备 11010802027423号
京公网安备 11010802027423号