当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrolysis of Gaseous CO2 to CO in a Flow Cell with a Bipolar Membrane
ACS Energy Letters ( IF 22.0 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acsenergylett.7b01017 Danielle A. Salvatore 1 , David M. Weekes 2 , Jingfu He 2 , Kevan E. Dettelbach 2 , Yuguang C. Li 3 , Thomas E. Mallouk 3 , Curtis P. Berlinguette 1, 2, 4
ACS Energy Letters ( IF 22.0 ) Pub Date : 2017-12-19 00:00:00 , DOI: 10.1021/acsenergylett.7b01017 Danielle A. Salvatore 1 , David M. Weekes 2 , Jingfu He 2 , Kevan E. Dettelbach 2 , Yuguang C. Li 3 , Thomas E. Mallouk 3 , Curtis P. Berlinguette 1, 2, 4
Affiliation
|
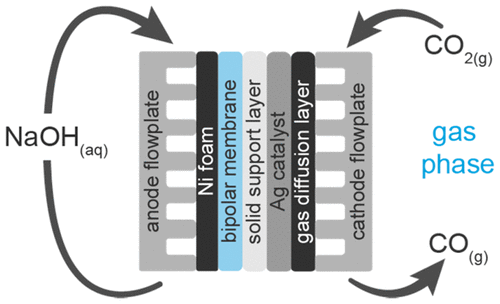
The conversion of CO2 to CO is demonstrated in an electrolyzer flow cell containing a bipolar membrane at current densities of 200 mA/cm2 with a Faradaic efficiency of 50%. Electrolysis was carried out by delivering gaseous CO2 at the cathode with a silver catalyst integrated in a carbon-based gas diffusion layer. Nonprecious nickel foam in a strongly alkaline electrolyte (1 M NaOH) was used to mediate the anode reaction. While a configuration where the anode and cathode were separated by only a bipolar membrane was found to be unfavorable for robust CO2 reduction, a modified configuration with a solid-supported aqueous layer inserted between the silver-based catalyst layer and the bipolar membrane enhanced the cathode selectivity for CO2 reduction to CO. We report higher current densities (200 mA/cm2) than previously reported for gas-phase CO2 to CO electrolysis and demonstrate the dependence of long-term stability on adequate hydration of the CO2 inlet stream.
中文翻译:

带有双极膜的流通池中气态CO 2到CO的电解
在包含双极膜的电解池流通池中,以200 mA / cm 2的电流密度和法拉第效率为50%证明了CO 2向CO的转化。电解是通过在阴极处输送气态CO 2以及集成在碳基气体扩散层中的银催化剂来进行的。使用强碱性电解液(1 M NaOH)中的非贵金属泡沫镍来介导阳极反应。虽然发现阳极和阴极仅被双极性膜隔开的配置不利于坚固的CO 2还原后,在银基催化剂层和双极性膜之间插入了固体支撑的水层,从而获得了改进的构型,从而提高了将CO 2还原为CO的阴极选择性。我们报道了更高的电流密度(200 mA / cm 2)用于气相CO 2到CO的电解,并证明了长期稳定性对CO 2入口物流充分水合的依赖性。
更新日期:2017-12-19
中文翻译:

带有双极膜的流通池中气态CO 2到CO的电解
在包含双极膜的电解池流通池中,以200 mA / cm 2的电流密度和法拉第效率为50%证明了CO 2向CO的转化。电解是通过在阴极处输送气态CO 2以及集成在碳基气体扩散层中的银催化剂来进行的。使用强碱性电解液(1 M NaOH)中的非贵金属泡沫镍来介导阳极反应。虽然发现阳极和阴极仅被双极性膜隔开的配置不利于坚固的CO 2还原后,在银基催化剂层和双极性膜之间插入了固体支撑的水层,从而获得了改进的构型,从而提高了将CO 2还原为CO的阴极选择性。我们报道了更高的电流密度(200 mA / cm 2)用于气相CO 2到CO的电解,并证明了长期稳定性对CO 2入口物流充分水合的依赖性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号