当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reactivity of Criegee Intermediates toward Carbon Dioxide
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1021/acs.jpclett.7b03154 Yen-Hsiu Lin,Kaito Takahashi,Jim Jr-Min Lin
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2017-12-22 00:00:00 , DOI: 10.1021/acs.jpclett.7b03154 Yen-Hsiu Lin,Kaito Takahashi,Jim Jr-Min Lin
|
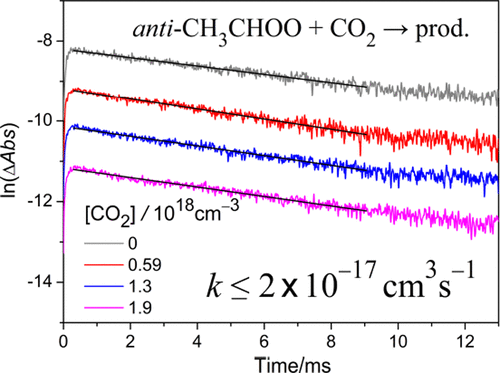
Recent theoretical work by Kumar and Francisco suggested that the high reactivity of Criegee intermediates (CIs) could be utilized for designing efficient carbon capture technologies. Because the anti-CH3CHOO + CO2 reaction has the lowest barrier in their study, we chose to investigate it experimentally. We probed anti-CH3CHOO with its strong UV absorption at 365 nm and measured the rate coefficient to be ≤2 × 10–17 cm3 molecule–1 s–1 at 298 K, which is consistent with our theoretical value of 2.1 × 10–17 cm3 molecule–1 s–1 at the QCISD(T)/CBS//B3LYP/6-311+G(2d,2p) level but inconsistent with their results obtained at the M06-2X/aug-cc-pVTZ level, which tends to underestimate the barrier heights. The experimental result indicates that the reaction of a Criegee intermediate with atmospheric CO2 (400 ppmv) would be inefficient (keff < 0.2 s–1) and cannot compete with other decay processes of Criegee intermediates like reactions with water vapor (∼103 s–1) or thermal decomposition (∼102 s–1).
中文翻译:

Criegee中间体对二氧化碳的反应活性
Kumar和Francisco的最新理论工作表明,Criegee中间体(CIs)的高反应活性可用于设计有效的碳捕集技术。因为抗-CH 3 CHOO + CO 2反应在他们的研究中具有最低的障碍,所以我们选择进行实验研究。我们在365 nm处检测到具有强紫外线吸收的抗-CH 3 CHOO,并在298 K下测得的速率系数为≤2×10 –17 cm 3分子–1 s –1,这与我们的理论值2.1× 10 –17 cm 3 分子–1 sQCISD(T)/ CBS // B3LYP / 6-311 + G(2d,2p)级别为–1,但与M06-2X / aug-cc-pVTZ级别获得的结果不一致,这往往会低估障碍高度。实验结果表明,Criegee中间体与大气CO 2(400 ppmv)的反应效率低(k eff <0.2 s –1),并且不能与其他Criegee中间体的衰变过程竞争,例如与水蒸气的反应(〜10 3)。 s –1)或热分解(〜10 2 s –1)。
更新日期:2017-12-22
中文翻译:

Criegee中间体对二氧化碳的反应活性
Kumar和Francisco的最新理论工作表明,Criegee中间体(CIs)的高反应活性可用于设计有效的碳捕集技术。因为抗-CH 3 CHOO + CO 2反应在他们的研究中具有最低的障碍,所以我们选择进行实验研究。我们在365 nm处检测到具有强紫外线吸收的抗-CH 3 CHOO,并在298 K下测得的速率系数为≤2×10 –17 cm 3分子–1 s –1,这与我们的理论值2.1× 10 –17 cm 3 分子–1 sQCISD(T)/ CBS // B3LYP / 6-311 + G(2d,2p)级别为–1,但与M06-2X / aug-cc-pVTZ级别获得的结果不一致,这往往会低估障碍高度。实验结果表明,Criegee中间体与大气CO 2(400 ppmv)的反应效率低(k eff <0.2 s –1),并且不能与其他Criegee中间体的衰变过程竞争,例如与水蒸气的反应(〜10 3)。 s –1)或热分解(〜10 2 s –1)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号