当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Glycosylation Promotes the Random Coil to Helix Transition in a Region of a Protist Skp1 Associated with F-Box Binding
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-28 00:00:00 , DOI: 10.1021/acs.biochem.7b01033 Xianzhong Xu 1 , Alexander Eletsky 1 , M. Osman Sheikh 1 , James H. Prestegard 1 , Christopher M. West 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-28 00:00:00 , DOI: 10.1021/acs.biochem.7b01033 Xianzhong Xu 1 , Alexander Eletsky 1 , M. Osman Sheikh 1 , James H. Prestegard 1 , Christopher M. West 1
Affiliation
|
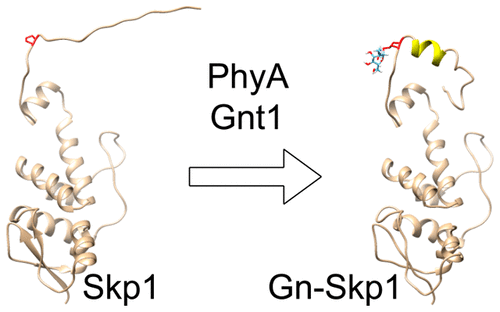
Cullin-ring-ligases mediate protein polyubiquitination, a signal for degradation in the 26S proteasome. The CRL1 class consists of Skp1/cullin-1/F-box protein/Rbx1 (SCF) complexes that cyclically associate with ubiquitin-E2 to build the polyubiquitin chain. Within the SCF complex, the 162-amino acid DdSkp1 from Dictyostelium bridges cullin-1 with an F-box protein (FBP), the specificity factor for substrate selection. The hydroxylation-dependent glycosylation of Pro143 of DdSkp1 by a pentasaccharide forms the basis of a novel O2-sensing mechanism in the social amoeba Dictyostelium and other protists. Previous evidence indicated that glycosylation promotes increased α-helical content correlating with enhanced interaction with three F-box proteins. To localize these differences, we used nuclear magnetic resonance (NMR) methods to compare nonglycosylated DdSkp1 and a glycoform with a single GlcNAc sugar (Gn-DdSkp1). We report NMR assignments of backbone 1HN, 15N, 13Cα, and 13CO nuclei as well as side-chain 13Cβ and methyl 13C/1H nuclei of Ile(δ1), Leu, and Val in both unmodified DdSkp1 and Gn-DdSkp1. The random coil index and 15N{1H} HNOE indicate that the C-terminal region, which forms a helix–loop–helix motif centered on Pro143 at the crystallographically defined binding interface with F-box domains, remains dynamic in both DdSkp1 and Gn-DdSkp1. Chemical shifts indicate that the variation of conformation in Gn-DdSkp1, relative to DdSkp1, is limited to this region and characterized by increased helical fold. Extension of the glycan chain results in further changes, also limited to this region. Thus, glycosylation may control F-box protein interactions via a local effect on DdSkp1 conformation, by a mechanism that may be general to many unicellular eukaryotes.
中文翻译:

糖基化促进与F-Box结合相关的Protist Skp1区域中的随机螺旋向螺旋转变。
Cullin-ring-ligases介导蛋白质多泛素化,这是26S蛋白酶体降解的信号。CRL1类由Skp1 / cullin-1 / F-box蛋白/ Rbx1(SCF)复合物组成,这些复合物与泛素E2循环缔合以构建聚泛素链。在SCF复合物中,来自盘基网柄菌的162个氨基酸的DdSkp1将cullin-1与F-box蛋白(FBP)(底物选择的特异性因子)桥接。由五糖DdSkp1的Pro143的羟基化,糖基化依赖性形成一种新的O的基础2 -sensing机构在社会变形虫盘基网柄和其他生物。先前的证据表明,糖基化促进与三个F-box蛋白相互作用增强相关的α-螺旋含量的增加。为了定位这些差异,我们使用核磁共振(NMR)方法比较了非糖基化DdSkp1和具有单个GlcNAc糖(Gn-DdSkp1)的糖型。我们报告的骨干NMR分配1 ħ Ñ,15 N,13 Ç α,和13个CO核以及侧链13 Ç β和甲基13 C / 1在两个岛(δ1),亮氨酸h的核,和Val未修改的DdSkp1和Gn-DdSkp1。随机线圈指数和15 N {1 H} HNOE表明,C末端区域在DdSkp1和Gn-DdSkp1中均保持动态,该区域在晶体学定义的结合界面上以Pro143为中心形成螺旋-环-螺旋基序。化学位移表明,相对于DdSkp1,Gn-DdSkp1构象的变化仅限于此区域,并以螺旋折叠增加为特征。聚糖链的延伸导致进一步的变化,也限于该区域。因此,糖基化可以通过对许多单细胞真核生物通用的机制,通过对DdSkp1构象的局部作用来控制F-box蛋白相互作用。
更新日期:2017-12-28
中文翻译:

糖基化促进与F-Box结合相关的Protist Skp1区域中的随机螺旋向螺旋转变。
Cullin-ring-ligases介导蛋白质多泛素化,这是26S蛋白酶体降解的信号。CRL1类由Skp1 / cullin-1 / F-box蛋白/ Rbx1(SCF)复合物组成,这些复合物与泛素E2循环缔合以构建聚泛素链。在SCF复合物中,来自盘基网柄菌的162个氨基酸的DdSkp1将cullin-1与F-box蛋白(FBP)(底物选择的特异性因子)桥接。由五糖DdSkp1的Pro143的羟基化,糖基化依赖性形成一种新的O的基础2 -sensing机构在社会变形虫盘基网柄和其他生物。先前的证据表明,糖基化促进与三个F-box蛋白相互作用增强相关的α-螺旋含量的增加。为了定位这些差异,我们使用核磁共振(NMR)方法比较了非糖基化DdSkp1和具有单个GlcNAc糖(Gn-DdSkp1)的糖型。我们报告的骨干NMR分配1 ħ Ñ,15 N,13 Ç α,和13个CO核以及侧链13 Ç β和甲基13 C / 1在两个岛(δ1),亮氨酸h的核,和Val未修改的DdSkp1和Gn-DdSkp1。随机线圈指数和15 N {1 H} HNOE表明,C末端区域在DdSkp1和Gn-DdSkp1中均保持动态,该区域在晶体学定义的结合界面上以Pro143为中心形成螺旋-环-螺旋基序。化学位移表明,相对于DdSkp1,Gn-DdSkp1构象的变化仅限于此区域,并以螺旋折叠增加为特征。聚糖链的延伸导致进一步的变化,也限于该区域。因此,糖基化可以通过对许多单细胞真核生物通用的机制,通过对DdSkp1构象的局部作用来控制F-box蛋白相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号