当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fluoroethylene Carbonate as a Directing Agent in Amorphous Silicon Anodes: Electrolyte Interface Structure Probed by Sum Frequency Vibrational Spectroscopy and Ab Initio Molecular Dynamics
Nano Letters ( IF 10.8 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.nanolett.7b04688 Yonatan Horowitz 1, 2 , Hui-Ling Han 1, 2 , Fernando A. Soto 3 , Walter T. Ralston 1, 2 , Perla B. Balbuena 3 , Gabor A. Somorjai 1, 2
Nano Letters ( IF 10.8 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.nanolett.7b04688 Yonatan Horowitz 1, 2 , Hui-Ling Han 1, 2 , Fernando A. Soto 3 , Walter T. Ralston 1, 2 , Perla B. Balbuena 3 , Gabor A. Somorjai 1, 2
Affiliation
|
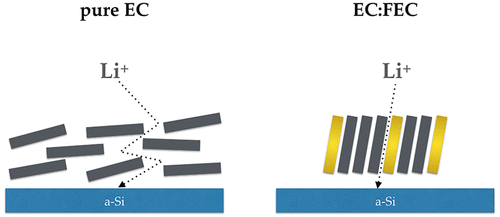
Fluorinated compounds are added to carbonate-based electrolyte solutions in an effort to create a stable solid electrolyte interphase (SEI). The SEI mitigates detrimental electrolyte redox reactions taking place on the anode’s surface upon applying a potential in order to charge (discharge) the lithium (Li) ion battery. The need for a stable SEI is dire when the anode material is silicon as silicon cracks due to its expansion and contraction upon lithiation and delithiation (charge–discharge) cycles, consequently limiting the cyclability of a silicon-based battery. Here we show the molecular structures for ethylene carbonate (EC): fluoroethylene carbonate (FEC) solutions on silicon surfaces by sum frequency generation (SFG) vibrational spectroscopy, which yields vibrational spectra of molecules at interfaces and by ab initio molecular dynamics (AIMD) simulations at open circuit potential. Our AIMD simulations and SFG spectra indicate that both EC and FEC adsorb to the amorphous silicon (a-Si) through their carbonyl group (C═O) oxygen atom with no further desorption. We show that FEC additives induce the reorientation of EC molecules to create an ordered, up-right orientation of the electrolytes on the Si surface. We suggest that this might be helpful for Li diffusion under applied potential. Furthermore, FEC becomes the dominant species at the a-Si surface as the FEC concentration increases above 20 wt %. Our finding at open circuit potential can now initiate additive design to not only act as a sacrificial compound but also to produce a better suited SEI for the use of silicon anodes in the Li-ion vehicular industry.
中文翻译:

碳酸氟乙烯酯作为非晶硅阳极中的定向剂:和频振动光谱法和从头算分子动力学探讨的电解质界面结构
将氟化化合物添加到碳酸盐基电解质溶液中,以创建稳定的固体电解质中间相(SEI)。SEI缓解了在施加电势以对锂(Li)离子电池充电(放电)时在阳极表面发生的有害电解质氧化还原反应。当负极材料是硅时,由于其在锂化和脱锂(充放电)循环时的膨胀和收缩,硅破裂时,对稳定SEI的需求非常迫切,从而限制了硅基电池的可循环性。在这里,我们通过求和频率生成(SFG)振动光谱法,在硅表面上显示了碳酸亚乙酯(EC):碳酸氟亚乙酯(FEC)溶液的分子结构,它会在界面处产生分子的振动光谱,并在开路电势下通过从头算分子动力学(AIMD)模拟来产生分子。我们的AIMD模拟和SFG光谱表明,EC和FEC都通过其羰基(C = O)氧原子吸附到非晶硅(a-Si),而没有进一步的解吸。我们表明,FEC添加剂会诱导EC分子重新取向,从而在Si表面上形成有序的,直立的电解质取向。我们建议这可能有助于在应用电势下锂的扩散。此外,当FEC浓度增加到20 wt%以上时,FEC成为a-Si表面的主要物种 我们的AIMD模拟和SFG光谱表明,EC和FEC都通过其羰基(C = O)氧原子吸附到非晶硅(a-Si),而没有进一步的解吸。我们表明,FEC添加剂会诱导EC分子重新取向,从而在Si表面上形成有序的,直立的电解质取向。我们建议这可能有助于在应用电势下锂的扩散。此外,当FEC浓度增加到20 wt%以上时,FEC成为a-Si表面的主要物种 我们的AIMD模拟和SFG光谱表明,EC和FEC都通过其羰基(C = O)氧原子吸附到非晶硅(a-Si),而没有进一步的解吸。我们表明,FEC添加剂会诱导EC分子重新取向,从而在Si表面上形成有序的,直立的电解质取向。我们建议这可能有助于在应用电势下锂的扩散。此外,当FEC浓度增加到20 wt%以上时,FEC成为a-Si表面的主要物种。我们在开路电势下的发现现在可以启动添加剂设计,使其不仅充当牺牲性化合物,而且还可以生产出更适合SEI的锂离子电池汽车工业中使用的硅阳极。
更新日期:2018-01-02
中文翻译:

碳酸氟乙烯酯作为非晶硅阳极中的定向剂:和频振动光谱法和从头算分子动力学探讨的电解质界面结构
将氟化化合物添加到碳酸盐基电解质溶液中,以创建稳定的固体电解质中间相(SEI)。SEI缓解了在施加电势以对锂(Li)离子电池充电(放电)时在阳极表面发生的有害电解质氧化还原反应。当负极材料是硅时,由于其在锂化和脱锂(充放电)循环时的膨胀和收缩,硅破裂时,对稳定SEI的需求非常迫切,从而限制了硅基电池的可循环性。在这里,我们通过求和频率生成(SFG)振动光谱法,在硅表面上显示了碳酸亚乙酯(EC):碳酸氟亚乙酯(FEC)溶液的分子结构,它会在界面处产生分子的振动光谱,并在开路电势下通过从头算分子动力学(AIMD)模拟来产生分子。我们的AIMD模拟和SFG光谱表明,EC和FEC都通过其羰基(C = O)氧原子吸附到非晶硅(a-Si),而没有进一步的解吸。我们表明,FEC添加剂会诱导EC分子重新取向,从而在Si表面上形成有序的,直立的电解质取向。我们建议这可能有助于在应用电势下锂的扩散。此外,当FEC浓度增加到20 wt%以上时,FEC成为a-Si表面的主要物种 我们的AIMD模拟和SFG光谱表明,EC和FEC都通过其羰基(C = O)氧原子吸附到非晶硅(a-Si),而没有进一步的解吸。我们表明,FEC添加剂会诱导EC分子重新取向,从而在Si表面上形成有序的,直立的电解质取向。我们建议这可能有助于在应用电势下锂的扩散。此外,当FEC浓度增加到20 wt%以上时,FEC成为a-Si表面的主要物种 我们的AIMD模拟和SFG光谱表明,EC和FEC都通过其羰基(C = O)氧原子吸附到非晶硅(a-Si),而没有进一步的解吸。我们表明,FEC添加剂会诱导EC分子重新取向,从而在Si表面上形成有序的,直立的电解质取向。我们建议这可能有助于在应用电势下锂的扩散。此外,当FEC浓度增加到20 wt%以上时,FEC成为a-Si表面的主要物种。我们在开路电势下的发现现在可以启动添加剂设计,使其不仅充当牺牲性化合物,而且还可以生产出更适合SEI的锂离子电池汽车工业中使用的硅阳极。


























 京公网安备 11010802027423号
京公网安备 11010802027423号