当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Molecular Rotor-Based Halo-Tag Ligand Enables a Fluorogenic Proteome Stress Sensor to Detect Protein Misfolding in Mildly Stressed Proteome
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00763 Matthew Fares 1 , Yinghao Li 1 , Yu Liu 1 , Kun Miao 1 , Zi Gao 1 , Yufeng Zhai 1 , Xin Zhang 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00763 Matthew Fares 1 , Yinghao Li 1 , Yu Liu 1 , Kun Miao 1 , Zi Gao 1 , Yufeng Zhai 1 , Xin Zhang 1
Affiliation
|
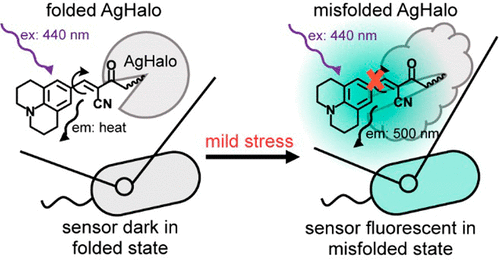
Cellular stress leads to disruption of protein homeostasis (proteostasis) that is associated with global misfolding and aggregation of the endogenous proteome. Monitoring stress-induced proteostasis deficiency remains one of the major technical challenges facing established sensors of this process. Available sensors use solvatochromic fluorophores to detect protein aggregation in forms of soluble oligomers or insoluble aggregates when cells are subjected to severe stress conditions. Misfolded monomers induced by mild stresses, however, remain largely invisible to these sensors. Here, we describe a fluorogenic proteome stress sensor by conjugating a fluorescent molecular rotor with a metastable Halo-tag protein domain that contains a K73T mutation (named AgHalo hereinafter). In nonstressed cells, the AaHalo sensor remains largely folded and the AgHalo•ligand conjugate is fluorescent dark in the folded state. Under various stress conditions, the AgHalo sensor has been established to form both soluble and insoluble aggregates along with metastable proteins of the endogenous cellular proteome. Thus, the AgHalo•ligand conjugate fluoresces strongly when the sensor forms misfolded monomers (a 16-fold increase) or aggregates in both soluble and insoluble forms (a 20-fold increase). Compared to the solvatochromic fluorophore-based sensor, we demonstrate that the molecular rotor-based sensor not only is more effective in detecting mild proteome stress that induces primarily misfolding conformations, but also exhibits a higher fluorescence signal in detecting more severe proteome stress that involves protein aggregates. Thus, the conjugation of a fluorescent molecular rotor to AgHalo further improves the capacity of this sensor to detect conditions of proteome stress. This work highlights the utility of molecular rotor-based fluorophores in direct visualization of the protein aggregation cascade in live cells, providing new methodologies for real-time analyses of cellular proteostasis upon exposure to different types of stress conditions.
中文翻译:

基于分子转子的Halo-Tag配体使荧光蛋白质组应力传感器能够检测轻度应力蛋白质组中的蛋白质错误折叠。
细胞压力导致蛋白质稳态(蛋白稳态)的破坏,这与内源性蛋白质组的整体错误折叠和聚集有关。监测压力诱导的蛋白稳态缺乏症仍然是该过程中已建立的传感器所面临的主要技术挑战之一。当细胞受到严峻的压力条件时,可用的传感器使用溶剂变色荧光团来检测可溶性寡聚体或不溶性聚集体形式的蛋白质聚集。但是,由轻度应力引起的错叠单体在很大程度上仍对这些传感器不可见。在这里,我们通过将荧光分子转子与包含K73T突变的亚稳态Halo-tag蛋白结构域(以下称为AgHalo)缀合来描述荧光蛋白组应力传感器。在无压力的细胞中,AaHalo传感器在很大程度上保持折叠状态,而AgHalo•配体偶联物在折叠状态下呈荧光暗。在各种压力条件下,已经建立了AgHalo传感器,以与内源细胞蛋白质组的亚稳态蛋白一起形成可溶性和不可溶的聚集体。因此,当传感器形成错误折叠的单体(增加16倍)或可溶和不溶形式的聚集体(增加20倍)时,AgHalo•配体共轭物会发出强烈的荧光。与基于溶剂化变色团的荧光基团的传感器相比,我们证明基于分子转子的传感器不仅在检测主要引起错误折叠构象的轻度蛋白质组应激方面更有效,而且在检测涉及蛋白质的更严重蛋白质组应激时表现出更高的荧光信号聚集体。因此,荧光分子转子与AgHalo的共轭进一步提高了该传感器检测蛋白质组应力条件的能力。这项工作强调了基于分子转子的荧光团在活细胞中蛋白质聚集级联反应的直接可视化中的实用性,为暴露于不同类型压力条件下的细胞蛋白稳态实时分析提供了新的方法。
更新日期:2018-01-02
中文翻译:

基于分子转子的Halo-Tag配体使荧光蛋白质组应力传感器能够检测轻度应力蛋白质组中的蛋白质错误折叠。
细胞压力导致蛋白质稳态(蛋白稳态)的破坏,这与内源性蛋白质组的整体错误折叠和聚集有关。监测压力诱导的蛋白稳态缺乏症仍然是该过程中已建立的传感器所面临的主要技术挑战之一。当细胞受到严峻的压力条件时,可用的传感器使用溶剂变色荧光团来检测可溶性寡聚体或不溶性聚集体形式的蛋白质聚集。但是,由轻度应力引起的错叠单体在很大程度上仍对这些传感器不可见。在这里,我们通过将荧光分子转子与包含K73T突变的亚稳态Halo-tag蛋白结构域(以下称为AgHalo)缀合来描述荧光蛋白组应力传感器。在无压力的细胞中,AaHalo传感器在很大程度上保持折叠状态,而AgHalo•配体偶联物在折叠状态下呈荧光暗。在各种压力条件下,已经建立了AgHalo传感器,以与内源细胞蛋白质组的亚稳态蛋白一起形成可溶性和不可溶的聚集体。因此,当传感器形成错误折叠的单体(增加16倍)或可溶和不溶形式的聚集体(增加20倍)时,AgHalo•配体共轭物会发出强烈的荧光。与基于溶剂化变色团的荧光基团的传感器相比,我们证明基于分子转子的传感器不仅在检测主要引起错误折叠构象的轻度蛋白质组应激方面更有效,而且在检测涉及蛋白质的更严重蛋白质组应激时表现出更高的荧光信号聚集体。因此,荧光分子转子与AgHalo的共轭进一步提高了该传感器检测蛋白质组应力条件的能力。这项工作强调了基于分子转子的荧光团在活细胞中蛋白质聚集级联反应的直接可视化中的实用性,为暴露于不同类型压力条件下的细胞蛋白稳态实时分析提供了新的方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号