Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Monoclonal Antibody Interfaces: Dilatation Mechanics and Bubble Coalescence
Langmuir ( IF 3.9 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acs.langmuir.7b03790 Aadithya Kannan 1, 2 , Ian C. Shieh 1, 2 , Danielle L. Leiske 1, 2 , Gerald G. Fuller 1, 2
Langmuir ( IF 3.9 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acs.langmuir.7b03790 Aadithya Kannan 1, 2 , Ian C. Shieh 1, 2 , Danielle L. Leiske 1, 2 , Gerald G. Fuller 1, 2
Affiliation
|
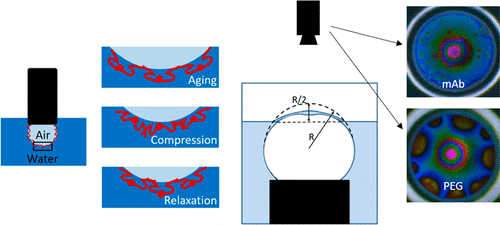
Monoclonal antibodies (mAbs) are proteins that uniquely identify targets within the body, making them well-suited for therapeutic applications. However, these amphiphilic molecules readily adsorb onto air–solution interfaces where they tend to aggregate. We investigated two mAbs with different propensities to aggregate at air–solution interfaces. The understanding of the interfacial rheological behavior of the two mAbs is crucial in determining their aggregation tendency. In this work, we performed interfacial stress relaxation studies under compressive step strain using a custom-built dilatational rheometer. The dilatational relaxation modulus was determined for these viscoelastic interfaces. The initial value and the equilibrated value of relaxation modulus were larger in magnitude for the mAb with a higher tendency to aggregate in response to interfacial stress. We also performed single-bubble coalescence experiments using a custom-built dynamic fluid-film interferometer (DFI). The bubble coalescence times also correlated to the mAbs aggregation propensity and interfacial viscoelasticity. To study the influence of surfactants in mAb formulations, polyethylene glycol (PEG) was chosen as a model surfactant. In the mixed mAb/PEG system, we observed that the higher aggregating mAb coadsorbed with PEG and formed domains at the interface. In contrast, for the other mAb, PEG entirely covered the interface at the concentrations studied. We studied the mobility of the interfaces, which was manifested by the presence or the lack of Marangoni stresses. These dynamics were strongly correlated with the interfacial viscoelasticity of the mAbs. The influence of competitive destabilization in affecting the bubble coalescence times for the mixed mAb/PEG systems was also studied.
中文翻译:

单克隆抗体的接口:扩散力学和气泡合并。
单克隆抗体(mAb)是可唯一识别体内靶标的蛋白质,使其非常适合治疗应用。但是,这些两亲分子很容易吸附到空气-溶液界面上,在这些界面上它们易于聚集。我们研究了两种具有不同倾向在空气-溶液界面聚集的单克隆抗体。了解两个mAb的界面流变行为对于确定其聚集趋势至关重要。在这项工作中,我们使用定制的膨胀流变仪在压缩阶跃应变下进行了界面应力松弛研究。确定这些粘弹性界面的膨胀松弛模量。对于mAb,弛豫模量的初始值和平衡值的大小较大,响应于界面应力,其趋于聚集的趋势更高。我们还使用定制的动态液膜干涉仪(DFI)进行了单气泡合并实验。气泡的聚结时间还与mAb的聚集倾向和界面粘弹性有关。为了研究表面活性剂在mAb配方中的影响,选择了聚乙二醇(PEG)作为模型表面活性剂。在混合的mAb / PEG系统中,我们观察到较高的聚集mAb与PEG共吸附,并在界面处形成结构域。相反,对于其他mAb,PEG在研究浓度下完全覆盖了界面。我们研究了接口的移动性,这是通过存在或缺乏Marangoni压力来体现的。这些动力学与mAb的界面粘弹性密切相关。还研究了竞争性不稳定因素对混合mAb / PEG系统气泡合并时间的影响。
更新日期:2018-01-03
中文翻译:

单克隆抗体的接口:扩散力学和气泡合并。
单克隆抗体(mAb)是可唯一识别体内靶标的蛋白质,使其非常适合治疗应用。但是,这些两亲分子很容易吸附到空气-溶液界面上,在这些界面上它们易于聚集。我们研究了两种具有不同倾向在空气-溶液界面聚集的单克隆抗体。了解两个mAb的界面流变行为对于确定其聚集趋势至关重要。在这项工作中,我们使用定制的膨胀流变仪在压缩阶跃应变下进行了界面应力松弛研究。确定这些粘弹性界面的膨胀松弛模量。对于mAb,弛豫模量的初始值和平衡值的大小较大,响应于界面应力,其趋于聚集的趋势更高。我们还使用定制的动态液膜干涉仪(DFI)进行了单气泡合并实验。气泡的聚结时间还与mAb的聚集倾向和界面粘弹性有关。为了研究表面活性剂在mAb配方中的影响,选择了聚乙二醇(PEG)作为模型表面活性剂。在混合的mAb / PEG系统中,我们观察到较高的聚集mAb与PEG共吸附,并在界面处形成结构域。相反,对于其他mAb,PEG在研究浓度下完全覆盖了界面。我们研究了接口的移动性,这是通过存在或缺乏Marangoni压力来体现的。这些动力学与mAb的界面粘弹性密切相关。还研究了竞争性不稳定因素对混合mAb / PEG系统气泡合并时间的影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号