当前位置:
X-MOL 学术
›
Photochem. Photobiol. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photoacidity as a tool to rationalize excited state intramolecular proton transfer reactivity in flavonols†
Photochemical & Photobiological Sciences ( IF 3.1 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1039/c7pp00334j Luís Gustavo Teixeira Alves Duarte 1, 2, 3, 4, 5 , José Carlos Germino 5, 6, 7, 8 , Cláudia de Ávila Braga 1, 2, 3, 4, 5 , Cristina Aparecida Barboza 9, 10, 11, 12 , Teresa Dib Zambon Atvars 5, 6, 7, 8 , Fabiano da Silveira Santos 1, 2, 3, 4, 5 , Fabiano Severo Rodembusch 1, 2, 3, 4, 5
Photochemical & Photobiological Sciences ( IF 3.1 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1039/c7pp00334j Luís Gustavo Teixeira Alves Duarte 1, 2, 3, 4, 5 , José Carlos Germino 5, 6, 7, 8 , Cláudia de Ávila Braga 1, 2, 3, 4, 5 , Cristina Aparecida Barboza 9, 10, 11, 12 , Teresa Dib Zambon Atvars 5, 6, 7, 8 , Fabiano da Silveira Santos 1, 2, 3, 4, 5 , Fabiano Severo Rodembusch 1, 2, 3, 4, 5
Affiliation
|
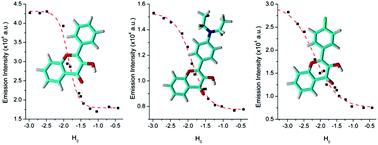
This work presents the determination of acidic strengths at the electronic ground and excited states (pKa and 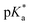 ) of three flavonol derivatives using electronic absorption and fluorescence emission spectroscopy. The differences of the pKa and
) of three flavonol derivatives using electronic absorption and fluorescence emission spectroscopy. The differences of the pKa and 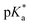 values were successfully correlated with the molecular structures according to the substitution pattern at the flavonol structure (hydrogen, diethylamino or fluoro moieties). In order to obtain more information about the observed photoacidity of these superacids, geometry optimizations and excitation energy calculations were performed at the CAM-B3LYP/6-311++G(d,p) level for their neutral, protonated and deprotonated species.
values were successfully correlated with the molecular structures according to the substitution pattern at the flavonol structure (hydrogen, diethylamino or fluoro moieties). In order to obtain more information about the observed photoacidity of these superacids, geometry optimizations and excitation energy calculations were performed at the CAM-B3LYP/6-311++G(d,p) level for their neutral, protonated and deprotonated species.
中文翻译:

光酸度是合理化黄酮醇中激发态分子内质子转移反应性的工具†
这项工作提出了使用电子吸收和荧光发射光谱法测定三种黄酮醇衍生物在电子基态和激发态(p K a和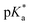 )的酸强度的方法。根据黄酮醇结构(氢,二乙氨基或氟部分)上的取代模式,p K a和
)的酸强度的方法。根据黄酮醇结构(氢,二乙氨基或氟部分)上的取代模式,p K a和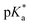 值的差异已成功地与分子结构相关。为了获得有关观察到的这些超强酸的光酸度的更多信息,在其中性,质子化和去质子化物质的CAM-B3LYP / 6-311 ++ G(d,p)水平上进行了几何优化和激发能计算。
值的差异已成功地与分子结构相关。为了获得有关观察到的这些超强酸的光酸度的更多信息,在其中性,质子化和去质子化物质的CAM-B3LYP / 6-311 ++ G(d,p)水平上进行了几何优化和激发能计算。
更新日期:2017-12-18
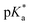 ) of three flavonol derivatives using electronic absorption and fluorescence emission spectroscopy. The differences of the pKa and
) of three flavonol derivatives using electronic absorption and fluorescence emission spectroscopy. The differences of the pKa and 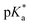 values were successfully correlated with the molecular structures according to the substitution pattern at the flavonol structure (hydrogen, diethylamino or fluoro moieties). In order to obtain more information about the observed photoacidity of these superacids, geometry optimizations and excitation energy calculations were performed at the CAM-B3LYP/6-311++G(d,p) level for their neutral, protonated and deprotonated species.
values were successfully correlated with the molecular structures according to the substitution pattern at the flavonol structure (hydrogen, diethylamino or fluoro moieties). In order to obtain more information about the observed photoacidity of these superacids, geometry optimizations and excitation energy calculations were performed at the CAM-B3LYP/6-311++G(d,p) level for their neutral, protonated and deprotonated species.
中文翻译:

光酸度是合理化黄酮醇中激发态分子内质子转移反应性的工具†
这项工作提出了使用电子吸收和荧光发射光谱法测定三种黄酮醇衍生物在电子基态和激发态(p K a和
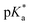 )的酸强度的方法。根据黄酮醇结构(氢,二乙氨基或氟部分)上的取代模式,p K a和
)的酸强度的方法。根据黄酮醇结构(氢,二乙氨基或氟部分)上的取代模式,p K a和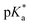 值的差异已成功地与分子结构相关。为了获得有关观察到的这些超强酸的光酸度的更多信息,在其中性,质子化和去质子化物质的CAM-B3LYP / 6-311 ++ G(d,p)水平上进行了几何优化和激发能计算。
值的差异已成功地与分子结构相关。为了获得有关观察到的这些超强酸的光酸度的更多信息,在其中性,质子化和去质子化物质的CAM-B3LYP / 6-311 ++ G(d,p)水平上进行了几何优化和激发能计算。


























 京公网安备 11010802027423号
京公网安备 11010802027423号