当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Lanthanides as Catalysts: Guided Ion Beam and Theoretical Studies of Sm+ + COS
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.jpca.7b09905 P. B. Armentrout 1 , Richard M Cox 1 , Brendan C. Sweeny 2 , Shaun G. Ard 2 , Nicholas S. Shuman 2 , Albert A. Viggiano 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.jpca.7b09905 P. B. Armentrout 1 , Richard M Cox 1 , Brendan C. Sweeny 2 , Shaun G. Ard 2 , Nicholas S. Shuman 2 , Albert A. Viggiano 2
Affiliation
|
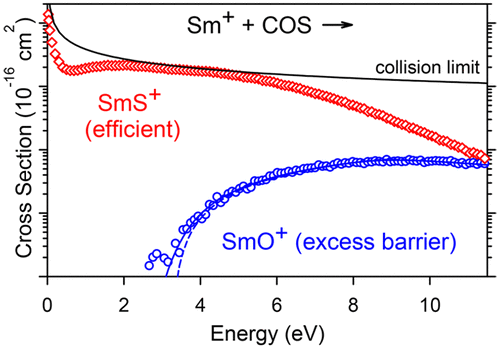
Reactions of samarium cations with carbonyl sulfide are examined using a guided ion beam tandem mass spectrometer and a variable temperature selected ion flow tube apparatus. Formation of SmS+ + CO is observed in both instruments with a kinetic energy and temperature dependence demonstrating a barrierless reaction occurring with an efficiency of 26 ± 9%. Formation of SmO+ + CS is also observed at high kinetic energies and exhibits a threshold determined as 2.81 ± 0.32 eV, substantially higher than expected from known thermochemistry. The potential energy surfaces for these reactions along sextet and octet spin surfaces are also examined theoretically at the MP2 and CCSD(T) levels. The observed barrier for oxidation is shown to likely correspond to the energy of the crossing between surfaces corresponding to the ground state electronic configuration of Sm+ (8F,4f66s1) and an excited surface having two electrons in the valence space (excluding 4f), which are needed to form the strong SmO+ bond. In contrast, the S–CO bond is activated much more readily because this crossing occurs at much lower energies. This result is attributed to the much weaker S–CO bond energy as well as the ability of sulfur to bind effectively at different angles. Although both reactions are spin-forbidden, evidence for a more efficient spin-allowed process is also observed in the SmS+ + CO cross section.
中文翻译:

镧系元素作为催化剂:引导离子束和Sm + + COS的理论研究
a离子与羰基硫的反应用导向离子束串联质谱仪和可变温度选择的离子流管设备进行检查。在这两种仪器中均观察到SmS + + CO的形成,并且具有动能和温度依赖性,这表明无障碍反应的发生效率为26±9%。SmO +的形成在高动能下也观察到+ CS,并且其阈值确定为2.81±0.32 eV,大大高于已知热化学的预期值。从理论上也在MP2和CCSD(T)级别上检查了沿着六面体和八面体自旋表面的这些反应的势能面。已显示观察到的氧化势垒可能对应于与Sm +(8 F,4f 6 6s 1)的基态电子构型相对应的表面和在价态空间中具有两个电子的受激表面之间的交叉能量4f),这是形成强SmO +所必需的键。相反,S-CO键更容易被激活,因为这种交叉发生在低得多的能量上。该结果归因于S-CO键能弱得多,以及硫以不同角度有效结合的能力。尽管两个反应都是自旋禁止的,但在SmS + + CO横截面中也观察到了更有效的自旋允许过程的证据。
更新日期:2018-01-12
中文翻译:

镧系元素作为催化剂:引导离子束和Sm + + COS的理论研究
a离子与羰基硫的反应用导向离子束串联质谱仪和可变温度选择的离子流管设备进行检查。在这两种仪器中均观察到SmS + + CO的形成,并且具有动能和温度依赖性,这表明无障碍反应的发生效率为26±9%。SmO +的形成在高动能下也观察到+ CS,并且其阈值确定为2.81±0.32 eV,大大高于已知热化学的预期值。从理论上也在MP2和CCSD(T)级别上检查了沿着六面体和八面体自旋表面的这些反应的势能面。已显示观察到的氧化势垒可能对应于与Sm +(8 F,4f 6 6s 1)的基态电子构型相对应的表面和在价态空间中具有两个电子的受激表面之间的交叉能量4f),这是形成强SmO +所必需的键。相反,S-CO键更容易被激活,因为这种交叉发生在低得多的能量上。该结果归因于S-CO键能弱得多,以及硫以不同角度有效结合的能力。尽管两个反应都是自旋禁止的,但在SmS + + CO横截面中也观察到了更有效的自旋允许过程的证据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号