当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
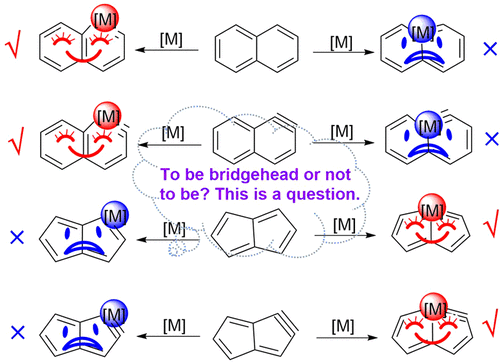
To Be Bridgehead or Not to Be? This is a Question of Metallabicycles on the Interplay between Aromaticity and Ring Strain
Organometallics ( IF 2.8 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.organomet.7b00758 Jingjing Wu 1 , Ke An 1 , Tingting Sun 1 , Jinglan Fan 1 , Jun Zhu 1
Organometallics ( IF 2.8 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.organomet.7b00758 Jingjing Wu 1 , Ke An 1 , Tingting Sun 1 , Jinglan Fan 1 , Jun Zhu 1
Affiliation
|
Transition-metal-containing metallaaromatics have attracted considerable interest from both experimental and computational chemists because they can display properties of both organometallic compounds and aromatic organic compounds. In general, the transition metal in a metallabicycle prefers a nonbridged position to the bridgehead one because of the larger ring strain caused by the rigidity in the bridgehead position, as exemplified by metallanaphthalene and metallanaphthalyne. On the contrary, the osmium atoms in recently synthesized osmapentalyne and osmapentalene are located at the bridgehead position. To probe the origin of the difference between these metallabicycles, we carried out density functional theory calculations. The metal-bridgehead osmanaphthalene and osmanaphthalyne are computed to be less stable by 17.9 and 26.3 kcal mol–1 than the non-metal-bridged ones, respectively. In addition, the metal-bridgehead osmapentalene and osmapentalyne are more stable by 11.8 and 22.8 kcal mol–1 than the non-metal-bridged isomers, respectively. Further study revealed that the ring strains in these paired isomers are comparable to each other. Thus, it is aromaticity rather than ring strain that determines the relative thermodynamic stabilities of these complexes.
中文翻译:

成为桥头堡还是不成为桥头堡?这是金属环对芳香性和环应变相互作用的一个问题
含过渡金属的金属芳香族化合物吸引了实验和计算化学家的极大兴趣,因为它们可以显示有机金属化合物和芳族有机化合物的特性。通常,金属环戊烷中的过渡金属比桥头优选过渡位置,这是因为桥头位置的刚性导致较大的环应变,例如金属镧系和金属酞菁系。相反,最近合成的osmapentalyne和osmapentalene中的the原子位于桥头位置。为了探究这些金属环之间差异的起源,我们进行了密度泛函理论计算。计算出金属桥头head精和精的稳定性较差,分别为17.9和26.3 kcal mol分别比非金属桥接器低–1。此外,金属桥头的osmapentalene和osmapentalyne分别比非金属桥联的异构体稳定11.8和22.8 kcal mol –1。进一步的研究表明,这些成对的异构体中的环应变彼此相当。因此,决定这些配合物的相对热力学稳定性的是芳香性而不是环应变。
更新日期:2017-12-15
中文翻译:

成为桥头堡还是不成为桥头堡?这是金属环对芳香性和环应变相互作用的一个问题
含过渡金属的金属芳香族化合物吸引了实验和计算化学家的极大兴趣,因为它们可以显示有机金属化合物和芳族有机化合物的特性。通常,金属环戊烷中的过渡金属比桥头优选过渡位置,这是因为桥头位置的刚性导致较大的环应变,例如金属镧系和金属酞菁系。相反,最近合成的osmapentalyne和osmapentalene中的the原子位于桥头位置。为了探究这些金属环之间差异的起源,我们进行了密度泛函理论计算。计算出金属桥头head精和精的稳定性较差,分别为17.9和26.3 kcal mol分别比非金属桥接器低–1。此外,金属桥头的osmapentalene和osmapentalyne分别比非金属桥联的异构体稳定11.8和22.8 kcal mol –1。进一步的研究表明,这些成对的异构体中的环应变彼此相当。因此,决定这些配合物的相对热力学稳定性的是芳香性而不是环应变。



























 京公网安备 11010802027423号
京公网安备 11010802027423号