当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reversible Twisting of Primary Amides via Ground State N–C(O) Destabilization: Highly Twisted Rotationally-Inverted Acyclic Amides
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-08 , DOI: 10.1021/jacs.7b11309 Guangrong Meng 1 , Shicheng Shi 1 , Roger Lalancette 1 , Roman Szostak 2 , Michal Szostak 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-08 , DOI: 10.1021/jacs.7b11309 Guangrong Meng 1 , Shicheng Shi 1 , Roger Lalancette 1 , Roman Szostak 2 , Michal Szostak 1
Affiliation
|
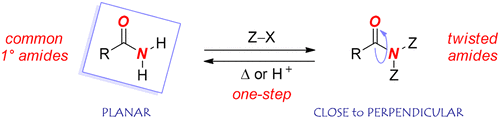
Since the seminal studies by Pauling in 1930s, planarity has become the defining characteristic of the amide bond. Planarity of amides has central implications for the reactivity and chemical properties of amides of relevance to a range of chemical disciplines. While the vast majority of amides are planar, nonplanarity has a profound effect on the properties of the amide bond, with the most common method to restrict the amide bond relying on the incorporation of the amide function into a rigid cyclic ring system. In a major departure from this concept, here, we report the first class of acyclic twisted amides that can be prepared, reversibly, from common primary amides in a single, operationally trivial step. Di-tert-butoxycarbonylation of the amide nitrogen atom yields twisted amides in which the amide bond exhibits nearly perpendicular twist. Full structural characterization of a range of electronically diverse compounds from this new class of twisted amides is reported. Through reactivity studies we demonstrate unusual properties of the amide bond, wherein selective cleavage of the amide bond can be achieved by a judicious choice of the reaction conditions. Through computational studies we evaluate structural and energetic details pertaining to the amide bond deformation. The ability to selectively twist common primary amides, in a reversible manner, has important implications for the design and application of the amide bond nonplanarity in structural chemistry, biochemistry and organic synthesis.
中文翻译:

通过基态 N-C(O) 失稳实现伯酰胺的可逆扭曲:高度扭曲的旋转反转无环酰胺
自 1930 年代 Pauling 进行开创性研究以来,平面性已成为酰胺键的定义特征。酰胺的平面性对与一系列化学学科相关的酰胺的反应性和化学性质具有重要意义。虽然绝大多数酰胺是平面的,但非平面性对酰胺键的性质有深远的影响,限制酰胺键的最常用方法依赖于将酰胺官能团结合到刚性环系统中。与此概念大相径庭,我们在这里报告了第一类无环扭曲酰胺,它们可以在一个简单的操作步骤中可逆地从常见的伯酰胺中制备。酰胺氮原子的二叔丁氧基羰基化产生扭曲的酰胺,其中酰胺键表现出几乎垂直的扭曲。报告了来自这类新型扭曲酰胺的一系列电子多样化化合物的完整结构表征。通过反应性研究,我们证明了酰胺键的不寻常特性,其中酰胺键的选择性裂解可以通过明智地选择反应条件来实现。通过计算研究,我们评估了与酰胺键变形有关的结构和能量细节。以可逆方式选择性扭曲常见伯酰胺的能力对酰胺键非平面性在结构化学、生物化学和有机合成中的设计和应用具有重要意义。通过反应性研究,我们证明了酰胺键的不寻常特性,其中酰胺键的选择性裂解可以通过明智地选择反应条件来实现。通过计算研究,我们评估了与酰胺键变形有关的结构和能量细节。以可逆方式选择性扭曲常见伯酰胺的能力对酰胺键非平面性在结构化学、生物化学和有机合成中的设计和应用具有重要意义。通过反应性研究,我们证明了酰胺键的不寻常特性,其中酰胺键的选择性裂解可以通过明智地选择反应条件来实现。通过计算研究,我们评估了与酰胺键变形有关的结构和能量细节。以可逆方式选择性扭曲常见伯酰胺的能力对酰胺键非平面性在结构化学、生物化学和有机合成中的设计和应用具有重要意义。
更新日期:2018-01-08
中文翻译:

通过基态 N-C(O) 失稳实现伯酰胺的可逆扭曲:高度扭曲的旋转反转无环酰胺
自 1930 年代 Pauling 进行开创性研究以来,平面性已成为酰胺键的定义特征。酰胺的平面性对与一系列化学学科相关的酰胺的反应性和化学性质具有重要意义。虽然绝大多数酰胺是平面的,但非平面性对酰胺键的性质有深远的影响,限制酰胺键的最常用方法依赖于将酰胺官能团结合到刚性环系统中。与此概念大相径庭,我们在这里报告了第一类无环扭曲酰胺,它们可以在一个简单的操作步骤中可逆地从常见的伯酰胺中制备。酰胺氮原子的二叔丁氧基羰基化产生扭曲的酰胺,其中酰胺键表现出几乎垂直的扭曲。报告了来自这类新型扭曲酰胺的一系列电子多样化化合物的完整结构表征。通过反应性研究,我们证明了酰胺键的不寻常特性,其中酰胺键的选择性裂解可以通过明智地选择反应条件来实现。通过计算研究,我们评估了与酰胺键变形有关的结构和能量细节。以可逆方式选择性扭曲常见伯酰胺的能力对酰胺键非平面性在结构化学、生物化学和有机合成中的设计和应用具有重要意义。通过反应性研究,我们证明了酰胺键的不寻常特性,其中酰胺键的选择性裂解可以通过明智地选择反应条件来实现。通过计算研究,我们评估了与酰胺键变形有关的结构和能量细节。以可逆方式选择性扭曲常见伯酰胺的能力对酰胺键非平面性在结构化学、生物化学和有机合成中的设计和应用具有重要意义。通过反应性研究,我们证明了酰胺键的不寻常特性,其中酰胺键的选择性裂解可以通过明智地选择反应条件来实现。通过计算研究,我们评估了与酰胺键变形有关的结构和能量细节。以可逆方式选择性扭曲常见伯酰胺的能力对酰胺键非平面性在结构化学、生物化学和有机合成中的设计和应用具有重要意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号