当前位置:
X-MOL 学术
›
Integr. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Generic maps of optimality reveal two chemomechanical coupling regimes for motor proteins: from F1-ATPase and kinesin to myosin and cytoplasmic dynein
Integrative Biology ( IF 2.5 ) Pub Date : 2018-01-03 , DOI: 10.1039/c7ib00142h Zhisong Wang 1, 2
Integrative Biology ( IF 2.5 ) Pub Date : 2018-01-03 , DOI: 10.1039/c7ib00142h Zhisong Wang 1, 2
Affiliation
|
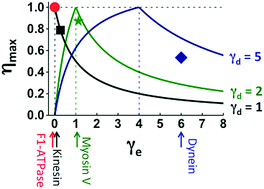
Many motor proteins achieve high efficiency for chemomechanical conversion, and single-molecule force-resisting experiments are a major tool to detect the chemomechanical coupling of efficient motors. Here, we introduce several quantitative relations that involve only parameters extracted from force-resisting experiments and offer new benchmarks beyond mere efficiency to judge the chemomechanical optimality or deficit of evolutionary remote motors on the same footing. The relations are verified by the experimental data from F1-ATPase, kinesin-1, myosin V and cytoplasmic dynein, which are representative members of four motor protein families. A double-fitting procedure yields the chemomechanical parameters that can be cross-checked for consistency. Using the extracted parameters, two generic maps of chemomechanical optimality are constructed on which motors across families can be quantitatively compared. The maps reveal two chemomechanical coupling regimes, one conducive to high efficiency and high directionality, and the other advantageous to force generation. Surprisingly, an F1 rotor and a kinesin-1 walker belong to the first regime despite their obvious evolutionary gap, while myosin V and cytoplasmic dynein follow the second regime. This analysis also predicts the symmetries of directional biases and heat productions for the motors, which impose constraints on their chemomechanical coupling and are open to future experimental tests. The verified relations, six in total, present a unified fitting framework to analyze force-resisting experiments. The generic maps of optimality, to which many more motors can be added in future, provide a rigorous method for a systematic cross-family comparison of motors to expose their evolutionary connections and mechanistic similarities.
中文翻译:

最佳性的通用图揭示了运动蛋白的两种化学机械耦合机制:从F 1 -ATPase和驱动蛋白到肌球蛋白和胞质动力蛋白
许多电机蛋白质可实现化学机械转换的高效率,而单分子抗力实验是检测高效电机化学机械耦合的主要工具。在这里,我们介绍了一些定量关系,这些关系仅涉及从抗力实验中提取的参数,并提供了超越单纯效率的新基准,可以在同一基础上判断进化型远程电动机的化学机械最佳性或缺陷。通过F 1的实验数据验证了这些关系-ATPase,kinesin-1,myosin V和胞质动力蛋白,是四个运动蛋白家族的代表成员。双重拟合过程可产生可交叉检查一致性的化学机械参数。使用提取的参数,构建了两个化学机械最佳化的通用图,可以在此两个图上对各个家族的电动机进行定量比较。这些图揭示了两种化学机械耦合方式,一种有利于高效率和高方向性,另一种有利于力的产生。令人惊讶的是F 1转子和kinesin-1沃克虽然具有明显的进化差距,但它们属于第一种治疗方案,而肌球蛋白V和细胞质动力蛋白则遵循第二种治疗方案。该分析还预测了电动机的方向偏差和热量产生的对称性,这些对称性对电动机的化学机械耦合施加了限制,并有待于将来的实验测试。经过验证的关系(总共六个)提供了一个统一的拟合框架来分析抗力实验。将来可以在其中添加更多电动机的通用最优图提供了一种严格的方法,可以对电动机进行系统的跨族比较,以揭示其进化联系和机械相似性。
更新日期:2018-01-03
中文翻译:

最佳性的通用图揭示了运动蛋白的两种化学机械耦合机制:从F 1 -ATPase和驱动蛋白到肌球蛋白和胞质动力蛋白
许多电机蛋白质可实现化学机械转换的高效率,而单分子抗力实验是检测高效电机化学机械耦合的主要工具。在这里,我们介绍了一些定量关系,这些关系仅涉及从抗力实验中提取的参数,并提供了超越单纯效率的新基准,可以在同一基础上判断进化型远程电动机的化学机械最佳性或缺陷。通过F 1的实验数据验证了这些关系-ATPase,kinesin-1,myosin V和胞质动力蛋白,是四个运动蛋白家族的代表成员。双重拟合过程可产生可交叉检查一致性的化学机械参数。使用提取的参数,构建了两个化学机械最佳化的通用图,可以在此两个图上对各个家族的电动机进行定量比较。这些图揭示了两种化学机械耦合方式,一种有利于高效率和高方向性,另一种有利于力的产生。令人惊讶的是F 1转子和kinesin-1沃克虽然具有明显的进化差距,但它们属于第一种治疗方案,而肌球蛋白V和细胞质动力蛋白则遵循第二种治疗方案。该分析还预测了电动机的方向偏差和热量产生的对称性,这些对称性对电动机的化学机械耦合施加了限制,并有待于将来的实验测试。经过验证的关系(总共六个)提供了一个统一的拟合框架来分析抗力实验。将来可以在其中添加更多电动机的通用最优图提供了一种严格的方法,可以对电动机进行系统的跨族比较,以揭示其进化联系和机械相似性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号