Journal of Catalysis ( IF 7.3 ) Pub Date : 2017-12-14 , DOI: 10.1016/j.jcat.2017.11.019 Chenwei Li , Franziska Hess , Igor Djerdj , Guangtao Chai , Yu Sun , Yanglong Guo , Bernd M. Smarsly , Herbert Over
|
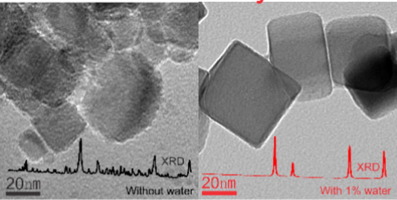
We studied the stability of CeO2 nano-cubes with preferentially (1 0 0)-oriented facets in the HCl oxidation reaction (Deacon process) for various reaction temperatures and the addition of small concentrations of water in the gas feed. For a reaction mixture HCl:O2 = 1:2 we find that CeO2 is substantially chlorinated below 380 °C, revealing a low catalytic activity. At 390 °C both the activity and the chlorination degree change abruptly: activity becomes high and chlorination is not detectable by XRD. The experimental results are rationalized by a kinetic model which allows us to study catalyst chlorination as a function of temperature and gas feed composition. The model predicts that chlorination sets in at the inlet of the catalyst bed and propagates then slowly along the catalyst bed towards the reactor outlet to fully chlorinate the CeO2 catalyst bed. This process has been confirmed by a dedicated experiment employing two separate catalyst layers, where only the first layer is shown to be chlorinated while exposed to the Deacon gas feed. Our model attributes the excessive chlorination at the reactor inlet to the absence of H2O in the gas feed, as the formation of H2O by the reaction of HCl with CeO2 is the prevailing driving force for catalyst chlorination. When running the Deacon process at 375 °C, the chlorination of CeO2 nano-cubes is efficiently suppressed by the addition of 1% water to the reaction mixture. This extrinsic stabilization of oxide catalysts towards chlorination by water is a general concept, which may enable the identification of new oxide materials that have previously been ruled out as Deacon catalysts due to their low stability under reaction conditions.
中文翻译:

在苛刻的HCl氧化反应中,水和高反应温度对CeO 2催化剂的稳定作用
我们研究了在各种反应温度和气体进料中添加少量水的情况下,在HCl氧化反应(迪肯工艺)中具有优先(1 0 0)取向的CeO 2纳米立方体的稳定性。对于HCl:O 2 = 1:2的反应混合物,我们发现CeO 2在低于380°C的温度下进行氯化,显示出较低的催化活性。在390°C时,活性和氯化度都会突然变化:活性变高,并且XRD无法检测到氯化。通过动力学模型使实验结果合理化,该动力学模型使我们能够研究催化剂氯化反应与温度和气体进料组成的关系。该模型预测,氯化作用会在催化剂床的入口处开始,然后缓慢地沿着催化剂床向反应器出口扩散,从而对CeO 2进行完全氯化。催化剂床。通过使用两个单独的催化剂层的专门实验已证实了该方法,其中仅第一层显示出在暴露于迪肯气体进料中时被氯化。我们的模型将反应器入口处的过量氯化归因于气体进料中不存在H 2 O,因为HCl与CeO 2反应形成的H 2 O是催化剂氯化的主要驱动力。在375°C下运行执事工艺时,CeO 2会氯化通过向反应混合物中添加1%的水可以有效地抑制纳米立方体。氧化物催化剂对水进行氯化的这种外在稳定是一个总的概念,由于在反应条件下其稳定性较低,因此可以识别以前被排除为迪肯催化剂的新型氧化物材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号