Molecular and Cellular Neuroscience ( IF 3.5 ) Pub Date : 2017-12-08 , DOI: 10.1016/j.mcn.2017.12.004 Andrey Khaitin , Mikhail Rudkovskii , Anatoly Uzdensky
|
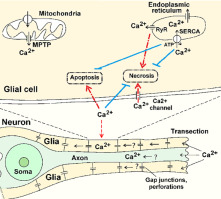
Severe nerve injury such as axotomy induces neuron degeneration and death of surrounding glial cells. Using a crayfish stretch receptor that consists of a single mechanoreceptor neuron enveloped by satellite glia, we showed that axotomy not only mechanically injures glial cells at the transection location, but also induces necrosis or apoptosis of satellite glial cells remote from the transection site. We studied Ca2+ role in spontaneous or axotomy-induced death of remote glial cells. Stretch receptors were isolated using the original technique that kept the neuron connected to the ventral cord ganglion (control preparations). Using Ca2+-sensitive fluorescence probe fluo-4, we showed Ca2+ accumulation in neuronal perikarion and glial envelope. Ca2+ gradually accumulated in glial cells after axotomy. In saline with triple Ca2+ concentration the axotomy-induced apoptosis of glial cells increased, but spontaneous or axotomy-induced necrosis was unexpectedly reduced. Saline with 1/3[Ca2+], oppositely, enhanced glial necrosis. Application of ionomycin, CdCl2, thapsigargin, and ryanodine showed the involvement of Ca2+ influx through ionic channels in the plasma membrane, inhibition of endoplasmic reticulum Ca2+-ATPase, and Ca2+ release from endoplasmic reticulum through ryanodine receptors in axotomy-induced glial necrosis. Apoptosis of glial cells surrounding axotomized neurons was promoted by ionomycin and thapsigargin. Possibly, other Ca2+ sources such as penetration through the plasma membrane contributed to axotomy-induced apoptosis and necrosis of remote glial cells. Thus, modulating different pathways that maintain calcium homeostasis, one can modulate axotomy-induced death of glial cells remote from the transection site.
中文翻译:

Ca 2+介导离体小龙虾机械感受器横切部位远处的卫星神经胶质细胞的轴突切开术引起的坏死和凋亡
严重的神经损伤(例如轴突切开术)会导致神经元变性和周围神经胶质细胞死亡。使用由小龙虾拉伸受体组成的小龙虾拉伸受体,由单个神经胶质细胞包裹着单个神经胶质神经元,我们证明了轴突切开术不仅在横断部位机械损伤神经胶质细胞,而且还诱导了远离横断部位的卫星神经胶质细胞的坏死或凋亡。我们研究了Ca 2+ 在自发性或轴突切开引起的远端神经胶质细胞死亡中的作用。使用保持神经元与腹神经节连接的原始技术分离拉伸受体(对照制剂)。使用Ca 2+敏感的荧光探针fluo-4,我们显示了神经元周围核和胶质包膜中的Ca 2+积累。钙轴切术后2+逐渐积聚在神经胶质细胞中。在具有两倍Ca 2+浓度的盐水中,轴突切开术引起的神经胶质细胞凋亡增加,但是自发性或轴突切开术引起的坏死却出乎意料地减少。相反,含1/3 [Ca 2+ ]的盐水可增强神经胶质坏死。施用离子霉素,CdCl 2,毒胡萝卜素和ryanodine表明,Ca 2+通过质膜上的离子通道参与了内流,抑制了内质网Ca 2+ -ATPase和Ca 2+轴突切开引起的神经胶质坏死中通过ryanodine受体从内质网释放。离子霉素和毒胡萝卜素促进轴突切除的神经元周围神经胶质细胞的凋亡。可能,其他Ca 2+来源(例如穿过质膜的渗透)也导致了轴突切开术诱导的细胞凋亡和远端神经胶质细胞的坏死。因此,调节维持钙稳态的不同途径,可以调节轴突切开术引起的远离横切部位的神经胶质细胞的死亡。



























 京公网安备 11010802027423号
京公网安备 11010802027423号