Journal of the American Society for Mass Spectrometry ( IF 3.2 ) Pub Date : 2017-08-30 , DOI: 10.1007/s13361-017-1778-9 Jacob W. McCabe 1 , Rajpal Vangala 1 , Laurence A. Angel 1
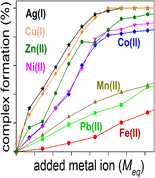
Methanobactin (Mb) from Methylosinus trichosporium OB3b is a member of a class of metal binding peptides identified in methanotrophic bacteria. Mb will selectively bind and reduce Cu(II) to Cu(I), and is thought to mediate the acquisition of the copper cofactor for the enzyme methane monooxygenase. These copper chelating properties of Mb make it potentially useful as a chelating agent for treatment of diseases where copper plays a role including Wilson’s disease, cancers, and neurodegenerative diseases. Utilizing traveling wave ion mobility-mass spectrometry (TWIMS), the competition for the Mb copper binding site from Ag(I), Pb(II), Co(II), Fe(II), Mn(II), Ni(II), and Zn(II) has been determined by a series of metal ion titrations, pH titrations, and metal ion displacement titrations. The TWIMS analyses allowed for the explicit identification and quantification of all the individual Mb species present during the titrations and measured their collision cross-sections and collision-induced dissociation patterns. The results showed Ag(I) and Ni(II) could irreversibly bind to Mb and not be effectively displaced by Cu(I), whereas Ag(I) could also partially displace Cu(I) from the Mb complex. At pH ≈ 6.5, the Mb binding selectivity follows the order Ag(I)≈Cu(I)>Ni(II)≈Zn(II)>Co(II)>>Mn(II)≈Pb(II)>Fe(II), and at pH 7.5 to 10.4 the order is Ag(I)>Cu(I)>Ni(II)>Co(II)>Zn(II)>Mn(II)≈Pb(II)>Fe(II). Breakdown curves of the disulfide reduced Cu(I) and Ag(I) complexes showed a correlation existed between their relative stability and their compact folded structure indicated by their CCS. Fluorescence spectroscopy, which allowed the determination of the binding constant, compared well with the TWIMS analyses, with the exception of the Ni(II) complex.
ᅟ
中文翻译:

甲烷菌素的结合选择性
毛果甲烷菌中的甲烷菌素(Mb)OB3b是在甲烷营养细菌中鉴定的一类金属结合肽的成员。Mb将选择性地将Cu(II)结合并还原为Cu(I),并被认为可以介导甲烷单加氧酶铜辅因子的获得。Mb的这些铜螯合性能使其潜在地用作治疗铜的疾病的螯合剂,其中威尔森氏病,癌症和神经退行性疾病起着铜的作用。利用行波离子迁移率质谱(TWIMS),Ag(I),Pb(II),Co(II),Fe(II),Mn(II),Ni(II)对Mb铜结合位点的竞争Zn(II)已通过一系列金属离子滴定,pH滴定和金属离子置换滴定确定。TWIMS分析可对滴定过程中存在的所有单个Mb物种进行明确的鉴定和定量,并测量其碰撞截面和碰撞诱导的解离模式。结果表明,Ag(I)和Ni(II)可以不可逆地结合到Mb上,而不能被Cu(I)有效地置换,而Ag(I)也可以从Mb配合物中部分置换Cu(I)。在pH≈6.5时,Mb的结合选择性遵循Ag(I)≈Cu(I)> Ni(II)≈Zn(II)> Co(II)>> Mn(II)≈Pb(II)> Fe( II),在pH 7.5至10.4的顺序为Ag(I)> Cu(I)> Ni(II)> Co(II)> Zn(II)> Mn(II)≈Pb(II)> Fe(II )。二硫化物还原的Cu(I)和Ag(I)配合物的分解曲线表明,它们的相对稳定性与由CCS指示的紧密折叠结构之间存在相关性。

ᅟ


























 京公网安备 11010802027423号
京公网安备 11010802027423号