当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Amyloid β (1–40) Toxicity Depends on the Molecular Contact between Phenylalanine 19 and Leucine 34
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1021/acschemneuro.7b00360 Alexander Korn 1 , Steffane McLennan 1 , Juliane Adler 1 , Martin Krueger 2 , Dayana Surendran 3 , Sudipta Maiti 3 , Daniel Huster 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1021/acschemneuro.7b00360 Alexander Korn 1 , Steffane McLennan 1 , Juliane Adler 1 , Martin Krueger 2 , Dayana Surendran 3 , Sudipta Maiti 3 , Daniel Huster 1
Affiliation
|
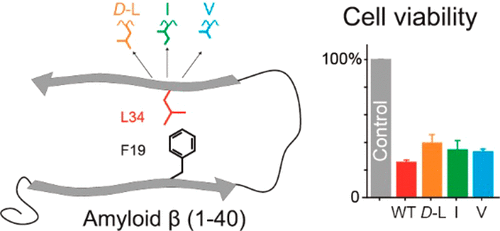
The formation of the hydrophobic contact between phenylalanine 19 (F19) and leucine 34 (L34) of amyloid β (1–40) (Aβ(1–40)) is known to be an important step in the fibrillation of Aβ(1–40) peptides. Mutations of this putatively early molecular contact were shown to strongly influence the toxicity of Aβ(1–40) (Das et al. (2015) ACS Chem. Neurosci. 6, 1290−1295). Any mutation of residue F19 completely abolished the toxicity of Aβ(1–40), suggesting that a proper F19–L34 contact is crucial also for the formation of transient oligomers. In this work, we investigate a series of isomeric substitutions of L34, namely, d-leucine, isoleucine, and valine, to study further details of this molecular contact. These replacements represent very minor alterations in the Aβ(1–40) structure posing the question how these alterations challenge the fibrillation kinetics, structure, dynamics, and toxicity of the Aβ(1–40) aggregates. Our work involves kinetic studies using thioflavin T, transmission electron microscopy, X-ray diffraction for the analysis of the fibril morphology, and nuclear magnetic resonance experiments for local structure and molecular dynamics investigations. Combined with cell toxicity assays of the mutated Aβ(1–40) peptides, the physicochemical and biological importance of the early folding contact between F19 and L34 in Aβ(1–40) is underlined. This implies that the F19–L34 contact influences a broad range of different processes including the initiation of fibrillation, oligomer stability, fibril elongation, local fibril structure, and dynamics and cellular toxicity. These processes do not only cover a broad range of diverse mechanisms, but also proved to be highly sensitive to minor modulations of this crucial contact. Furthermore, our work shows that the contact is not simply mediated by general hydrophobic interactions, but also depends on stereospecific mechanisms.
中文翻译:

淀粉样蛋白β(1–40)毒性取决于苯丙氨酸19和亮氨酸34之间的分子接触
已知淀粉样β(1–40)(Aβ(1–40))的苯丙氨酸19(F19)和亮氨酸34(L34)之间形成疏水接触,这是Aβ(1–40)原纤化的重要步骤)肽。此推定早期分子接触的突变显示强烈影响Aβ(1-40)(Das等人。(毒性2015)ACS化学式神经科学。 6,1290年至1295年)。残基F19的任何突变都完全消除了Aβ(1–40)的毒性,这表明正确的F19–L34接触对于瞬态低聚物的形成也至关重要。在这项工作中,我们研究了L34的一系列异构体取代,即d-亮氨酸,异亮氨酸和缬氨酸,以研究这种分子接触的更多细节。这些替代代表了Aβ(1–40)结构中很小的变化,提出了一个问题,即这些变化如何挑战Aβ(1–40)聚集体的原纤化动力学,结构,动力学和毒性。我们的工作涉及使用硫代黄素T的动力学研究,透射电子显微镜,X射线衍射(用于分析原纤维形态)以及核磁共振实验(用于局部结构和分子动力学研究)。结合突变的Aβ(1–40)肽的细胞毒性测定,强调了F19和L34在Aβ(1–40)中早期折叠接触的物理化学和生物学重要性。这意味着F19–L34接触会影响广泛的不同过程,包括纤维化的开始,低聚物稳定性,纤维原形伸长,局部纤维原状结构以及动力学和细胞毒性。这些过程不仅涵盖各种各样的机制,而且被证明对这种关键性接触的微小调制高度敏感。此外,我们的工作表明,接触不仅是由一般的疏水相互作用简单介导的,而且还取决于立体定向的机理。
更新日期:2017-12-12
中文翻译:

淀粉样蛋白β(1–40)毒性取决于苯丙氨酸19和亮氨酸34之间的分子接触
已知淀粉样β(1–40)(Aβ(1–40))的苯丙氨酸19(F19)和亮氨酸34(L34)之间形成疏水接触,这是Aβ(1–40)原纤化的重要步骤)肽。此推定早期分子接触的突变显示强烈影响Aβ(1-40)(Das等人。(毒性2015)ACS化学式神经科学。 6,1290年至1295年)。残基F19的任何突变都完全消除了Aβ(1–40)的毒性,这表明正确的F19–L34接触对于瞬态低聚物的形成也至关重要。在这项工作中,我们研究了L34的一系列异构体取代,即d-亮氨酸,异亮氨酸和缬氨酸,以研究这种分子接触的更多细节。这些替代代表了Aβ(1–40)结构中很小的变化,提出了一个问题,即这些变化如何挑战Aβ(1–40)聚集体的原纤化动力学,结构,动力学和毒性。我们的工作涉及使用硫代黄素T的动力学研究,透射电子显微镜,X射线衍射(用于分析原纤维形态)以及核磁共振实验(用于局部结构和分子动力学研究)。结合突变的Aβ(1–40)肽的细胞毒性测定,强调了F19和L34在Aβ(1–40)中早期折叠接触的物理化学和生物学重要性。这意味着F19–L34接触会影响广泛的不同过程,包括纤维化的开始,低聚物稳定性,纤维原形伸长,局部纤维原状结构以及动力学和细胞毒性。这些过程不仅涵盖各种各样的机制,而且被证明对这种关键性接触的微小调制高度敏感。此外,我们的工作表明,接触不仅是由一般的疏水相互作用简单介导的,而且还取决于立体定向的机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号