当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Benchmarking the Oxygen Reduction Electroactivity of First‐Row Transition‐Metal Oxide Clusters on Carbon Nanotubes
ChemElectroChem ( IF 4 ) Pub Date : 2017-12-27 , DOI: 10.1002/celc.201701215 Kuang-Hsu Wu 1, 2 , Mikaela Allen-Ankins 2 , Qingcong Zeng 1, 2 , Bingsen Zhang 3 , Jian Pan 1 , Jiayun Zhang 3 , Dang-Sheng Su 3, 4 , Ian R. Gentle 2 , Da-Wei Wang 1
ChemElectroChem ( IF 4 ) Pub Date : 2017-12-27 , DOI: 10.1002/celc.201701215 Kuang-Hsu Wu 1, 2 , Mikaela Allen-Ankins 2 , Qingcong Zeng 1, 2 , Bingsen Zhang 3 , Jian Pan 1 , Jiayun Zhang 3 , Dang-Sheng Su 3, 4 , Ian R. Gentle 2 , Da-Wei Wang 1
Affiliation
|
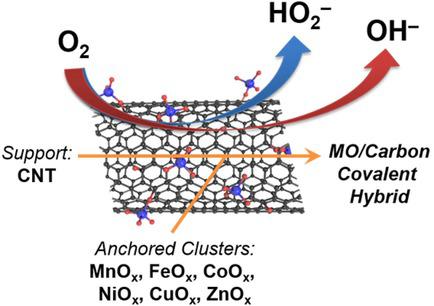
Transition‐metal oxide/nanocarbon hybrids are known to deliver a cooperative oxygen reduction reaction (ORR) with a synergistic effect of the hybrid interface. This work assesses a series of first‐row transition‐metal oxides in the form of surface‐confined clusters on carbon nanotubes, as a mimic of fully exposed hybrid interfaces, providing an activity benchmark for hybrid catalysts. Among the selected metal oxides, MnOx and CoOx are found to be the most active first‐row transition‐metal oxide clusters for the ORR, given their excellent peroxide‐reducing capability at low overpotential. FeOx and CuOx are also active for the same role, except their peroxide‐reducing activity is only activated with substantial overpotential. Other metal oxides (NiOx and ZnOx) do not appear to show any activity toward the ORR, even with an integrated interface as a source for the possible synergistic effect.
中文翻译:

基准测试碳纳米管上第一行过渡金属氧化物簇的氧还原电活性
已知过渡金属氧化物/纳米碳杂化物可产生协同的氧还原反应(ORR),并具有杂化物界面的协同作用。这项工作评估了一系列第一行过渡金属氧化物,以碳纳米管上表面受限簇的形式存在,模拟了完全暴露的杂化界面,为杂化催化剂提供了活性基准。在选定的金属氧化物中,发现MnO x和CoO x是ORR活性最高的第一行过渡金属氧化物簇,因为它们在低电势下具有出色的过氧化物还原能力。FeO x和CuO x它们也具有相同的作用,只是它们的减少过氧化物的活性仅在相当大的超电势下才被激活。其他金属氧化物(NiO x和ZnO x)似乎没有表现出对ORR的任何活性,即使使用集成界面作为可能产生协同作用的来源。
更新日期:2017-12-27
中文翻译:

基准测试碳纳米管上第一行过渡金属氧化物簇的氧还原电活性
已知过渡金属氧化物/纳米碳杂化物可产生协同的氧还原反应(ORR),并具有杂化物界面的协同作用。这项工作评估了一系列第一行过渡金属氧化物,以碳纳米管上表面受限簇的形式存在,模拟了完全暴露的杂化界面,为杂化催化剂提供了活性基准。在选定的金属氧化物中,发现MnO x和CoO x是ORR活性最高的第一行过渡金属氧化物簇,因为它们在低电势下具有出色的过氧化物还原能力。FeO x和CuO x它们也具有相同的作用,只是它们的减少过氧化物的活性仅在相当大的超电势下才被激活。其他金属氧化物(NiO x和ZnO x)似乎没有表现出对ORR的任何活性,即使使用集成界面作为可能产生协同作用的来源。



























 京公网安备 11010802027423号
京公网安备 11010802027423号