当前位置:
X-MOL 学术
›
J. Food Drug Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regulatory analysis on the medical use of ephedrine-related products in Taiwan
Journal of Food and Drug Analysis ( IF 3.6 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.jfda.2017.11.002 Wan-Nan Yu , Li-Hsuan Wang , Hui-Wen Cheng
Journal of Food and Drug Analysis ( IF 3.6 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.jfda.2017.11.002 Wan-Nan Yu , Li-Hsuan Wang , Hui-Wen Cheng
|
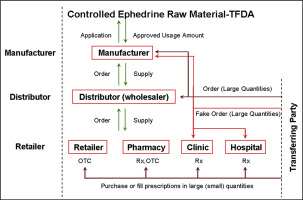
To prevent ephedrine-related products from being misused to produce amphetamine and/or its analogs, there's a need for more effective and achievable regulatory mechanisms for the health, police, investigational, prosecution and judiciary authorities in Taiwan. This review was conducted to evaluate the international and Taiwan's regulatory policies and management of medical ephedrine-related products through the corresponding information collected from international and Taiwan government agency authorities. The combat of illegal drugs should involve both supply and demand sides to be successful. Health authorities in Taiwan do not have the investigational power to manage the forbidden transformation, abusing and manufacture of the illegal drugs from ephedrine-related products. Take the judicial interventions in the United States and in Japan as the examples, the organizational cooperation in Taiwan can be one of the main key strategies to combat against illegal drugs from ephedrine-related products. It is necessary to integrate the judicial, police and health agencies to prevent the production of illegal drugs from the ephedrine-related products in Taiwan. The efforts and regulatory control measures should be integrated to speed up the collaboration between different government authorities. It might be achieved through reorganization involving Taiwan Food and Drug Administration.
更新日期:2018-04-01


























 京公网安备 11010802027423号
京公网安备 11010802027423号