Environmental Research ( IF 8.3 ) Pub Date : 2017-11-02 , DOI: 10.1016/j.envres.2017.10.045 Juanjuan Liu , Chong Dai , Yandi Hu
|
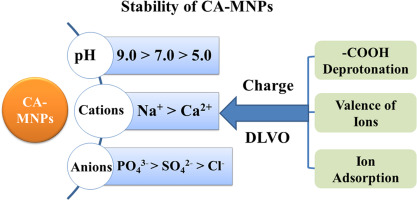
Improving the colloidal stability of magnetite nanoparticles (MNPs) is essential for their successful applications. In this study, the surface zeta potential and particle size evolutions of citric acid coated magnetite nanoparticles (CA-MNPs) were measured under varied aqueous conditions using dynamic light scattering (DLS). The effects of pH (5.0–9.0), ionic strength (IS), cations (Na+ and Ca2+), anions (phosphate, sulfate, and chloride) and humic acid on the aggregation behaviors of CA-MNPs were explored. Compared with bare MNPs, the stability of CA-MNPs were greatly improved over the typical pH range of natural aquatic environments (pH = 5.0–9.0), as the coated CA-MNPs were highly negatively charged over the pH range due to the low pKa1 value (3.13) of citrate acid. CA-MNPs were more stable in the presence of monovalent cation (Na+) compared with divalent cation (Ca2+), as Ca2+ could neutralize the surface charge of MNPs more significantly than Na+. In the presence of anions, the surface charges of CA-MNPs became more negative, and the stability of CA-MNPs followed the order: in phosphate > sulfate > chloride. The observed aggregation trend could be explained by the differences in the valences of the anions and their adsorption behaviors onto CA-MNPs, which altered the surface charges of CA-MNPs. The measured critical coagulation concentrations (CCC) values of CA-MNPs in these electrolyte solutions agreed well with Derjaguin–Landau–Verwey–Overbeek (DLVO) calculations. With the addition of Humic acid (HA), the aggregation of CA-MNPs was inhibited in all electrolyte solutions even with the critical coagulation concentrations. This is due to the adsorption of HA onto CA-MNPs, which enhanced the electrostatic and steric repulsive forces between CA-MNPs. Considering the good stability of CA-MNPs in solutions with varied pH and electrolyte compositions, as well as with the easy synthesis of CA-MNPs and their non-toxicity, this study suggested CA coating as a good strategy to increase the stability of MNPs.
中文翻译:

柠檬酸包覆的磁铁矿纳米颗粒的水聚集行为:pH,阳离子,阴离子和腐殖酸的影响
改善磁铁矿纳米粒子(MNP)的胶体稳定性对于其成功应用至关重要。在这项研究中,使用动态光散射(DLS)在变化的水性条件下测量了柠檬酸包覆的磁铁矿纳米颗粒(CA-MNPs)的表面zeta电位和粒度演变。探讨了pH(5.0–9.0),离子强度(IS),阳离子(Na +和Ca 2+),阴离子(磷酸根,硫酸根和氯离子)和腐殖酸对CA-MNPs聚集行为的影响。与裸露的MNPs相比,CA-MNPs在自然水生环境的典型pH范围(pH = 5.0–9.0)下的稳定性得到了极大的改善,因为涂膜的CA-MNPs在低pH范围内具有高负电荷ķ A1柠檬酸的值(3.13)。与二价阳离子(Ca 2+)相比,存在单价阳离子(Na +)时,CA-MNP更稳定,因为Ca 2+可以比Na +更有效地中和MNPs的表面电荷。在阴离子存在下,CA-MNPs的表面电荷变得更负,并且CA-MNPs的稳定性遵循以下顺序:磷酸盐>硫酸盐>氯化物。观察到的聚集趋势可以用阴离子的价数及其在CA-MNPs上的吸附行为的差异来解释,这改变了CA-MNPs的表面电荷。这些电解质溶液中CA-MNPs的临界凝结浓度(CCC)值与Derjaguin–Landau–Verwey–Overbeek(DLVO)计算非常吻合。通过添加腐殖酸(HA),即使在临界凝结浓度下,CA-MNP的聚集也能在所有电解质溶液中得到抑制。这是由于HA在CA-MNP上的吸附,从而增强了CA-MNP之间的静电和空间排斥力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号