Chemistry and Physics of Lipids ( IF 3.4 ) Pub Date : 2017-11-24 , DOI: 10.1016/j.chemphyslip.2017.11.015 Danijela Bakarić , Dražen Petrov , Yamuna Kunhi Mouvenchery , Stefan Heiβler , Chris Oostenbrink , Gabriele E. Schaumann
|
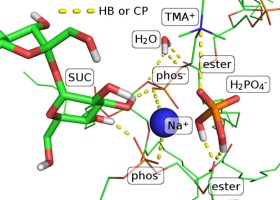
Disaccharides play an important role in survival of anhydrobiotic organisms during extreme environmental conditions. A key protection feature is their capability to form the hydrogen bond (HB) network in a similar fashion as the one made by water. Since various ions also affect the HB network in completely hydrated systems, it is of a great interest to understand how they impact preservation when incorporated in a disaccharide network. To address this, we employ a combination of experimental and modeling techniques to study behavior of multilamellar 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) liposomes freeze-dried with sucrose in presence of NaCl or NaH2PO4·H2O at various concentrations (0.01–1 M). Differential scanning calorimetry (DSC) was employed in order to determine the cooperative unit size (CUS), the number of lipid molecules that constitute a domain of cooperative motion in the liposome, and the melting temperature (Tm). In the absence of salt CUS was estimated to be 122 ± 12, whereas in the presence of NaCl CUS increases more (347 ± 34 for c = 1 M) than for NaH2PO4·H2O (193 ± 26 for 1 M). When it comes to Tm, the situation is reversed; NaCl induces increase by about 1 K, while NaH2PO4·H2O by about 10 K. These findings clearly demonstrate how different interaction forces−hydrogen bonding, charge pairing, and van der Waals interactions between acyl chains−affect CUS and Tm. Their interplay and contribution of particular interaction was further analyzed with molecular dynamics (MD) simulations. This analysis demonstrated that the HB network of DMPC and sucrose is partially disrupted in the presence of NaCl ions, and even to a greater extent in the case of NaH2PO4·H2O ions. Notably, H2PO4− ions outcompete and replace the sucrose molecules at the DMPC surface, which in turn alters the nature of the DMPC-surrounding interactions, from a weaker HB-dominated to a stronger CP-dominated interaction network.
中文翻译:

离子诱导的蔗糖网络修饰及其对冻干脂质体融化的影响。DSC和分子动力学研究
在极端环境条件下,二糖在水生生物的生存中起着重要作用。关键的保护功能是它们能够以类似于水的方式形成氢键(HB)网络的能力。由于各种离子也会影响完全水合系统中的HB网络,因此,了解将其掺入二糖网络时它们如何影响保藏意义重大。为了解决这个问题,我们采用的实验和建模技术来多层1,2-二肉豆蔻的学习行为的组合SN -glycero -3-磷酸胆碱(DMPC)的脂质体与蔗糖在NaCl或NaH的存在下冷冻干燥2 PO 4 ·高2O在各种浓度下(0.01-1 M)。使用差示扫描量热法(DSC)来确定协同单位大小(CUS),构成脂质体中协同运动域的脂质分子的数量以及熔化温度(T m)。在没有盐的情况下,CUS的估计值是122±12,而在有NaCl的情况下,CUS的增加值(c = 1 M时为347±34 )比NaH 2 PO 4 ·H 2 O(1 M时为193±26 )更大。)。当涉及到T m时,情况就相反了。NaCl诱导增加约1 K,而NaH 2 PO 4 ·H 2O约10K。这些发现清楚地表明,不同的相互作用力-氢键,电荷配对和酰基链之间的范德华相互作用-影响CUS和T m。它们的相互作用和特殊相互作用的贡献还通过分子动力学(MD)模拟进行了进一步分析。该分析表明,在存在NaCl离子的情况下,DMPC和蔗糖的HB网络被部分破坏,在NaH 2 PO 4 ·H 2 O离子的情况下甚至更大程度地破坏。值得注意的是,H 2 PO 4 - 离子竞争并取代了DMPC表面的蔗糖分子,从而改变了DMPC周围相互作用的性质,从较弱的HB为主到较强的CP为主的相互作用网络。



























 京公网安备 11010802027423号
京公网安备 11010802027423号