Nano Research ( IF 9.9 ) Pub Date : 2018-08-02 , DOI: 10.1007/s12274-017-1930-7 Aleksandr Kakinen , Jozef Adamcik , Bo Wang , Xinwei Ge , Raffaele Mezzenga , Thomas P. Davis , Feng Ding , Pu Chun Ke
Understanding how small molecules interface with amyloid fibrils at the nanoscale is of importance for developing therapeutic treatments against amyloid-based diseases. Here, we show for the first time that human islet amyloid polypeptides (IAPP) in the fibrillar form are polymorphic, ambidextrous, and possess multiple periodicities. Upon interfacing with the small molecule epigallocatechin gallate (EGCG), IAPP aggregation was rendered off-pathway and assumed a form with soft and disordered clusters, while mature IAPP fibrils displayed kinks and branching but conserved the twisted fibril morphology. These nanoscale phenomena resulted from competitive interactions between EGCG and the IAPP amyloidogenic region, as well as end capping of the fibrils by the small molecule. This information is crucial in delineating IAPP toxicity implicated in type 2 diabetes and for developing new inhibitors against amyloidogenesis.
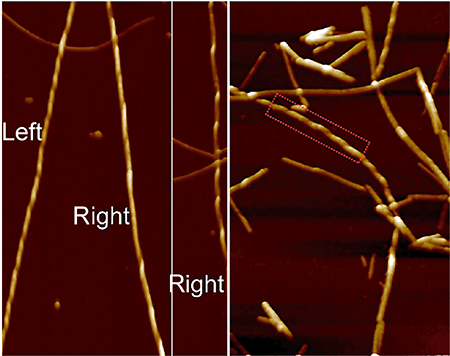
中文翻译:

纳米抑制多态性和灵巧的IAPP淀粉样蛋白聚集的小分子。
了解小分子如何在纳米级与淀粉样蛋白原纤维接触对于开发针对基于淀粉样蛋白的疾病的治疗方法非常重要。在这里,我们首次显示原纤维形式的人类胰岛淀粉样多肽(IAPP)具有多态性,灵巧性,并具有多个周期性。与小分子表没食子儿茶素没食子酸酯(EGCG)交界后,IAPP聚集体脱离了通路,呈现出柔软无序的簇状形式,而成熟的IAPP原纤维则表现出纽结和分支,但保留了扭曲的原纤维形态。这些纳米级现象是由EGCG和IAPP淀粉样蛋白生成区域之间的竞争性相互作用以及小分子对原纤维的封端所致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号