当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bioinspired total synthesis of tetrahydrofuran lignans by tandem nucleophilic addition/redox isomerization/oxidative coupling and cycloetherification reactions as key steps†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1039/c7ob02848b Pratap R. Jagtap 1, 2, 3 , Ivana Císařová 3, 4, 5, 6 , Ullrich Jahn 1, 2, 3
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1039/c7ob02848b Pratap R. Jagtap 1, 2, 3 , Ivana Císařová 3, 4, 5, 6 , Ullrich Jahn 1, 2, 3
Affiliation
|
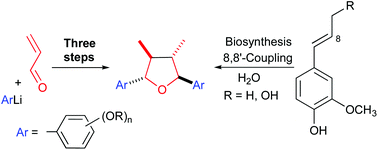
A very short three-step approach to trans,trans,trans-2,5-diaryl-3,4-dimethyltetrahydrofuran lignans is reported. The carbon skeleton is assembled in a single step based on an unprecedented tandem reaction consisting of 1,2-addition of aryllithium reagents to α,β-unsaturated aldehydes, ruthenium-catalyzed redox isomerization of the resulting alkoxides to enolates and their dimerization triggered by single electron oxidation. The resulting 2,3-dialkyl-1,4-diketones form with moderate to good d/l-diastereoselectivity and are transformed to the target tetrahydrofuran lignans by reduction and diastereoselective cycloetherification.
中文翻译:

串联亲核加成/氧化还原异构化/氧化偶联和环醚化反应的生物启发性全合成四氢呋喃木脂素是关键步骤†
据报道,反式,反式,反式-2,5-二芳基-3,4-二甲基四氢呋喃木脂素的非常短的三步法。碳骨架是基于空前的串联反应由一个步骤组装而成的,该串联反应由芳基锂试剂与α,β-不饱和醛的1,2-加成反应,钌催化的所得醇盐氧化还原异构化为烯醇化物以及它们的二聚反应引发。电子氧化。形成的2,3-二烷基-1,4-二酮具有中等至良好的d / l-非对映选择性,并通过还原和非对映选择性环醚化转化为目标四氢呋喃木脂素。
更新日期:2017-12-12
中文翻译:

串联亲核加成/氧化还原异构化/氧化偶联和环醚化反应的生物启发性全合成四氢呋喃木脂素是关键步骤†
据报道,反式,反式,反式-2,5-二芳基-3,4-二甲基四氢呋喃木脂素的非常短的三步法。碳骨架是基于空前的串联反应由一个步骤组装而成的,该串联反应由芳基锂试剂与α,β-不饱和醛的1,2-加成反应,钌催化的所得醇盐氧化还原异构化为烯醇化物以及它们的二聚反应引发。电子氧化。形成的2,3-二烷基-1,4-二酮具有中等至良好的d / l-非对映选择性,并通过还原和非对映选择性环醚化转化为目标四氢呋喃木脂素。


























 京公网安备 11010802027423号
京公网安备 11010802027423号