当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Indium Catalyzed Hydrofunctionalization of Styrene Derivatives Bearing a Hydroxy Group with Organosilicon Nucleophiles
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acs.joc.7b02739 Yuji Kita 1 , Tetsuji Yata 1 , Yoshihiro Nishimoto 1 , Makoto Yasuda 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acs.joc.7b02739 Yuji Kita 1 , Tetsuji Yata 1 , Yoshihiro Nishimoto 1 , Makoto Yasuda 1
Affiliation
|
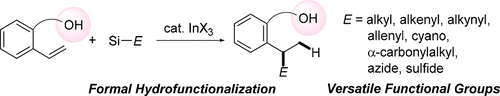
Hydrofunctionalization is one of the most important transformation reactions of alkenes. Herein, we describe the development of an indium-triiodide-catalyzed hydrofunctionalization of alkenes bearing a hydroxy group using various types of organosilicon nucleophiles. Indium triiodide was the most effective catalyst, whereas typical Lewis acids such as TiCl4, AlCl3, and BF3·OEt2 were ineffective. Many functional groups were successfully introduced, and these resulted in yields of 31–86%. Various styrene derivatives were also applicable to this reaction. Mechanistic investigation revealed that the present hydrofunctionalization proceeded through Brønsted acid-catalyzed intramolecular hydroalkoxylation of alkenes followed by InI3-catalyzed substitution reaction of cyclic ether intermediates.
中文翻译:

带有有机硅亲核试剂的带有羟基的苯乙烯衍生物的铟催化加氢官能化
加氢官能化是烯烃最重要的转化反应之一。在本文中,我们描述了使用各种类型的有机硅亲核试剂的三碘化铟催化带有羟基的烯烃的加氢官能化。三碘化铟是最有效的催化剂,而典型的路易斯酸(如TiCl 4,AlCl 3和BF 3 ·OEt 2)无效。成功引入了许多官能团,这些化合物的收率为31–86%。各种苯乙烯衍生物也适用于该反应。机理研究表明,目前的加氢官能化是通过布朗斯台德酸催化的烯烃分子内加氢烷氧基化反应进行的,然后是InI进行。环醚中间体的3-催化取代反应。
更新日期:2018-01-09
中文翻译:

带有有机硅亲核试剂的带有羟基的苯乙烯衍生物的铟催化加氢官能化
加氢官能化是烯烃最重要的转化反应之一。在本文中,我们描述了使用各种类型的有机硅亲核试剂的三碘化铟催化带有羟基的烯烃的加氢官能化。三碘化铟是最有效的催化剂,而典型的路易斯酸(如TiCl 4,AlCl 3和BF 3 ·OEt 2)无效。成功引入了许多官能团,这些化合物的收率为31–86%。各种苯乙烯衍生物也适用于该反应。机理研究表明,目前的加氢官能化是通过布朗斯台德酸催化的烯烃分子内加氢烷氧基化反应进行的,然后是InI进行。环醚中间体的3-催化取代反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号