当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Desymmetrizing Enantio- and Diastereoselective Selenoetherification through Supramolecular Catalysis
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acscatal.7b03510 Jie Yang See 1 , Hui Yang 1 , Yu Zhao 1 , Ming Wah Wong 1 , Zhihai Ke 2 , Ying-Yeung Yeung 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-02 00:00:00 , DOI: 10.1021/acscatal.7b03510 Jie Yang See 1 , Hui Yang 1 , Yu Zhao 1 , Ming Wah Wong 1 , Zhihai Ke 2 , Ying-Yeung Yeung 1, 2
Affiliation
|
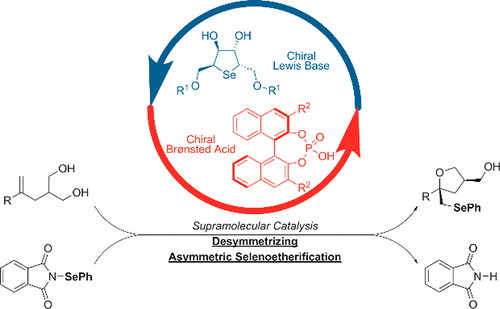
Selenofunctionalization is used for the introduction of aryl- or alkylseleno moieties, which can then be transformed into other functional groups (such as alkenes and carbonyls). However, asymmetric selenofunctionalization of unactivated olefins is often difficult to realize, as aryl- and alkylseleno cations rapidly interchange between olefinic partners. Recently, it has been demonstrated that Lewis bases, assisted by Brønsted acids, induce high levels of enantioselectivity in selenocyclization reactions. The Brønsted acid serves as an activator for the reaction. In this work, we demonstrate an asymmetric selenoetherification and desymmetrization of olefinic 1,3-diols, driven by a unique chiral pairing between a C2-symmetric cyclic selenide catalyst and a chiral Brønsted acid. The resulting substituted tetrahydrofurans contain a phenylselenoether handle and can be transformed into synthetically useful building blocks. A series of experimental and computational investigations suggest that the reaction proceeds via a supramolecular catalytic pathway.
中文翻译:

通过超分子催化解对称对映体和非对映体选择性硒醚化
硒代官能化用于引入芳基或烷基硒基部分,然后可以将其转化成其他官能团(例如烯烃和羰基)。但是,未活化的烯烃的不对称硒官能化通常难以实现,因为芳基和烷基硒化阳离子在烯烃配偶体之间迅速交换。最近,已证明在布朗斯台德酸的辅助下,路易斯碱在硒环化反应中诱导高水平的对映选择性。布朗斯台德酸作为反应的活化剂。在这项工作中,我们证明烯1,3-二醇的不对称selenoetherification和desymmetrization,由一个唯一的手性配对之间驱动Ç 2 -对称环状硒化物催化剂和手性布朗斯台德酸。所得的取代的四氢呋喃含有苯基硒醚手柄,可以转化为合成有用的结构单元。一系列实验和计算研究表明,该反应通过超分子催化途径进行。
更新日期:2018-01-02
中文翻译:

通过超分子催化解对称对映体和非对映体选择性硒醚化
硒代官能化用于引入芳基或烷基硒基部分,然后可以将其转化成其他官能团(例如烯烃和羰基)。但是,未活化的烯烃的不对称硒官能化通常难以实现,因为芳基和烷基硒化阳离子在烯烃配偶体之间迅速交换。最近,已证明在布朗斯台德酸的辅助下,路易斯碱在硒环化反应中诱导高水平的对映选择性。布朗斯台德酸作为反应的活化剂。在这项工作中,我们证明烯1,3-二醇的不对称selenoetherification和desymmetrization,由一个唯一的手性配对之间驱动Ç 2 -对称环状硒化物催化剂和手性布朗斯台德酸。所得的取代的四氢呋喃含有苯基硒醚手柄,可以转化为合成有用的结构单元。一系列实验和计算研究表明,该反应通过超分子催化途径进行。


























 京公网安备 11010802027423号
京公网安备 11010802027423号