当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Trends in Metallophilic Bonding in Pd–Zn and Pd–Cu Complexes
Organometallics ( IF 2.8 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acs.organomet.7b00748 Eno Paenurk 1 , Renana Gershoni-Poranne 1 , Peter Chen 1
Organometallics ( IF 2.8 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acs.organomet.7b00748 Eno Paenurk 1 , Renana Gershoni-Poranne 1 , Peter Chen 1
Affiliation
|
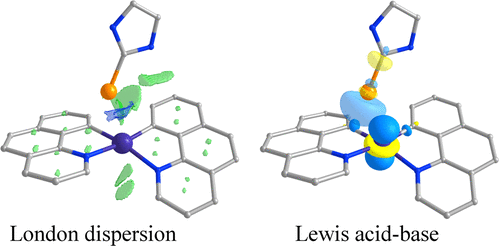
Metallophilic interactions stabilize the bond between closed-shell metal centers, which electrostatically repel one another. Since their introduction, the origin of these interactions has been argued to be either London dispersion forces or dative bonding, but as yet, there is no definitive answer. Insight into the nature of metallophilic bonding would provide the key for rational tuning of the stabilizing interaction, for example, in specific transmetalation transition states. We now report on a computational study focused on the metallophilic d8–d10 bond in recently published families of Pd(II)–Cu(I) and Pd(II)–Zn(II) heterobimetallic compexes. We show that dative bonding outweighs dispersion interaction in controlling the metallophilic bonding energy in the studied heterobimetallic complexes, and elucidate the governing orbital interactions.
中文翻译:

Pd-Zn和Pd-Cu络合物的亲金属键的趋势
亲金属相互作用稳定了闭壳金属中心之间的键,该中心彼此静电排斥。自从引入以来,这些相互作用的起源一直被认为是伦敦的分散力量或发展性的联系,但是到目前为止,还没有确切的答案。对嗜金属键性质的深入了解将为稳定相互作用的合理调节提供关键,例如在特定的过渡金属化过渡态下。我们现在报告一项针对嗜金属性d 8 –d 10的计算研究最近发表的Pd(II)-Cu(I)和Pd(II)-Zn(II)异双金属化合物家族中的碳键。我们表明,在控制异双金属配合物中的亲金属键合能量时,导数键合胜于色散相互作用,并阐明了支配性的轨道相互作用。
更新日期:2017-12-11
中文翻译:

Pd-Zn和Pd-Cu络合物的亲金属键的趋势
亲金属相互作用稳定了闭壳金属中心之间的键,该中心彼此静电排斥。自从引入以来,这些相互作用的起源一直被认为是伦敦的分散力量或发展性的联系,但是到目前为止,还没有确切的答案。对嗜金属键性质的深入了解将为稳定相互作用的合理调节提供关键,例如在特定的过渡金属化过渡态下。我们现在报告一项针对嗜金属性d 8 –d 10的计算研究最近发表的Pd(II)-Cu(I)和Pd(II)-Zn(II)异双金属化合物家族中的碳键。我们表明,在控制异双金属配合物中的亲金属键合能量时,导数键合胜于色散相互作用,并阐明了支配性的轨道相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号