当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quinazolinone-Based Anticancer Agents: Synthesis, Antiproliferative SAR, Antitubulin Activity, and Tubulin Co-crystal Structure
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01474 Wolfgang Dohle 1 , Fabrice L. Jourdan 2 , Grégory Menchon 3 , Andrea E. Prota 3 , Paul A. Foster 4, 5 , Pascoe Mannion 4, 5 , Ernest Hamel 6 , Mark P. Thomas 2 , Philip G. Kasprzyk 7 , Eric Ferrandis 8 , Michel O. Steinmetz 3, 9 , Mathew P. Leese 2 , Barry V. L. Potter 1, 2
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01474 Wolfgang Dohle 1 , Fabrice L. Jourdan 2 , Grégory Menchon 3 , Andrea E. Prota 3 , Paul A. Foster 4, 5 , Pascoe Mannion 4, 5 , Ernest Hamel 6 , Mark P. Thomas 2 , Philip G. Kasprzyk 7 , Eric Ferrandis 8 , Michel O. Steinmetz 3, 9 , Mathew P. Leese 2 , Barry V. L. Potter 1, 2
Affiliation
|
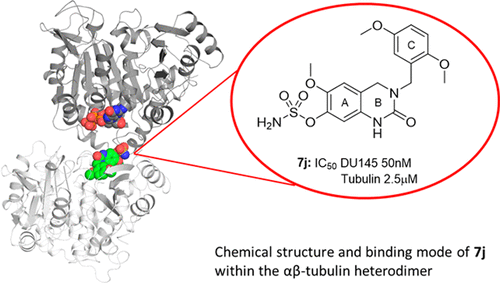
Quinazolinone-based anticancer agents were designed, decorated with functional groups from a 2-methoxyestradiol-based microtubule disruptor series, incorporating the aryl sulfamate motif of steroid sulfatase (STS) inhibitors. The steroidal AB-ring system was mimicked, favoring conformations with an N-2 substituent occupying D-ring space. Evaluation against breast and prostate tumor cell lines identified 7b with DU-145 antiproliferative activity (GI50 300 nM). A preliminary structure–activity relationship afforded compounds (e.g., 7j GI50 50 nM) with activity exceeding that of the parent. Both 7b and 7j inhibit tubulin assembly in vitro and colchicine binding, and 7j was successfully co-crystallized with the αβ-tubulin heterodimer as the first of its class, its sulfamate group interacting positively at the colchicine binding site. Microtubule destabilization by 7j is likely achieved by preventing the curved-to-straight conformational transition in αβ-tubulin. Quinazolinone sulfamates surprisingly showed weak STS inhibition. Preliminary in vivo studies in a multiple myeloma xenograft model for 7b showed oral activity, confirming the promise of this template.
中文翻译:

基于喹唑啉酮的抗癌药:合成,抗增殖SAR,抗微管蛋白活性和微管蛋白共晶体结构
设计了基于喹唑啉酮的抗癌剂,并修饰了基于2-甲氧基雌二醇的微管破坏剂系列的官能团,并结合了甾族硫酸酯酶(STS)抑制剂的芳基氨基磺酸酯基序。模拟甾体AB-环系统,有利于具有占据D-环空间的N -2取代基的构象。针对乳腺癌和前列腺肿瘤细胞系的评估确定了7b具有DU-145抗增殖活性(GI 50 300 nM)。初步的结构-活性关系提供了活性超过母体的化合物(例如7j GI 50 50 nM)。无论7B和7J抑制微管蛋白在体外的组装和秋水仙碱的结合,并成功地将7j与αβ-微管蛋白异二聚体共结晶,这是其首个类别,其氨基磺酸盐基团在秋水仙碱的结合位点呈正相互作用。7j引起的微管失稳可能是通过防止α-微管蛋白从弯曲到笔直的构象转变来实现的。氨基磺酸喹唑啉酮令人惊讶地显示出弱的STS抑制作用。在多发性骨髓瘤7b异种移植模型中的初步体内研究显示了口服活性,证实了该模板的前景。
更新日期:2018-01-08
中文翻译:

基于喹唑啉酮的抗癌药:合成,抗增殖SAR,抗微管蛋白活性和微管蛋白共晶体结构
设计了基于喹唑啉酮的抗癌剂,并修饰了基于2-甲氧基雌二醇的微管破坏剂系列的官能团,并结合了甾族硫酸酯酶(STS)抑制剂的芳基氨基磺酸酯基序。模拟甾体AB-环系统,有利于具有占据D-环空间的N -2取代基的构象。针对乳腺癌和前列腺肿瘤细胞系的评估确定了7b具有DU-145抗增殖活性(GI 50 300 nM)。初步的结构-活性关系提供了活性超过母体的化合物(例如7j GI 50 50 nM)。无论7B和7J抑制微管蛋白在体外的组装和秋水仙碱的结合,并成功地将7j与αβ-微管蛋白异二聚体共结晶,这是其首个类别,其氨基磺酸盐基团在秋水仙碱的结合位点呈正相互作用。7j引起的微管失稳可能是通过防止α-微管蛋白从弯曲到笔直的构象转变来实现的。氨基磺酸喹唑啉酮令人惊讶地显示出弱的STS抑制作用。在多发性骨髓瘤7b异种移植模型中的初步体内研究显示了口服活性,证实了该模板的前景。



























 京公网安备 11010802027423号
京公网安备 11010802027423号