当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the Chemical Mechanism for CO2(aq) and H+ in Aqueous Diamine Solutions - An Experimental Stopped-Flow Kinetic and 1H/13C NMR Study of Aqueous Solutions of N,N-Dimethylethylenediamine for Postcombustion CO2 Capture
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.est.7b05226 Bing Yu 1 , Lichun Li 1 , Hai Yu 1 , Marcel Maeder , Graeme Puxty 1 , Qi Yang 2 , Paul Feron 1 , William Conway 1 , Zuliang Chen
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1021/acs.est.7b05226 Bing Yu 1 , Lichun Li 1 , Hai Yu 1 , Marcel Maeder , Graeme Puxty 1 , Qi Yang 2 , Paul Feron 1 , William Conway 1 , Zuliang Chen
Affiliation
|
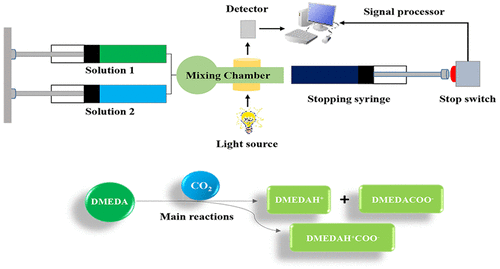
In an effort to advance the understanding of multiamine based CO2 capture process absorbents, we report here the determination of the kinetic and equilibrium constants for a simple linear diamine N,N-dimethylethylenediamine (DMEDA) via stopped-flow spectrophotometric kinetic measurements and 1H/13C NMR titrations at 25.0 °C. From the kinetic data, the formation of monocarbamic acid (DMEDACOOH) from the reaction of DMEDA with CO2(aq) is the dominant reaction at high pH > 9.0 (k7 = 6.99 × 103 M–1·s–1). Below this pH, the formation of protonated monocarbamic acid (DMEDACOOH2) via the pathway involving DMEDAH+ and CO2(aq) becomes active and contributes to the kinetics despite the 107-fold decrease in the rate constant between the two pathways. 1H and 13C NMR spectra as a function of decreasing pH (increasing HCl concentration) at 25.0 °C have been evaluated here to confirm the protonation events in DMEDA. Calculations of the respective DMEDA nitrogen partial charges have also been undertaken to support the NMR protonation study. A comparison of the DMEDA kinetic constants with the corresponding data for piperazine (PZ) reveals that despite the larger basicity of DMEDA, the enhanced and superior kinetic performance of PZ with CO2(aq) above its predicted Bronsted reactivity is not observed in DMEDA.
中文翻译:

二胺水溶液中CO 2(aq)和H +的化学机理的见解-N,N-二甲基乙二胺水溶液燃烧后CO 2捕集的实验停止流动力学和1 H / 13 C NMR研究
为了增进对基于多胺的CO 2捕集工艺吸收剂的理解,我们在此报告通过停止流分光光度法动力学测量和1 H确定简单线性二胺N,N-二甲基乙二胺(DMEDA)的动力学和平衡常数。/ 13在25.0℃下C NMR滴定。根据动力学数据,在高pH> 9.0(k 7 = 6.99×10 3 M –1 ·s –1)时,DMEDA与CO 2(aq)反应形成的单氨基甲酸(DMEDACOOH)是主要反应。低于此pH值,会形成质子化一氨基甲酸(DMEDACOOH 2)通过包含DMEDAH +和CO 2(aq)的途径变得活跃,尽管在两条途径之间的速率常数降低了107倍,但仍有助于动力学。1 H和13 C NMR光谱作为在25.0°C下pH值降低(HCl浓度增加)的函数进行了评估,以确认DMEDA中的质子化事件。还进行了相应DMEDA氮部分装料的计算,以支持NMR质子化研究。将DMEDA动力学常数与哌嗪(PZ)的相应数据进行比较后发现,尽管DMEDA的碱度较大,但CO 2可以增强PZ的动力学性能,使其具有优越的动力学性能。在DMEDA中未观察到(aq)高于其预期的布朗斯台德反应性。
更新日期:2018-01-05
中文翻译:

二胺水溶液中CO 2(aq)和H +的化学机理的见解-N,N-二甲基乙二胺水溶液燃烧后CO 2捕集的实验停止流动力学和1 H / 13 C NMR研究
为了增进对基于多胺的CO 2捕集工艺吸收剂的理解,我们在此报告通过停止流分光光度法动力学测量和1 H确定简单线性二胺N,N-二甲基乙二胺(DMEDA)的动力学和平衡常数。/ 13在25.0℃下C NMR滴定。根据动力学数据,在高pH> 9.0(k 7 = 6.99×10 3 M –1 ·s –1)时,DMEDA与CO 2(aq)反应形成的单氨基甲酸(DMEDACOOH)是主要反应。低于此pH值,会形成质子化一氨基甲酸(DMEDACOOH 2)通过包含DMEDAH +和CO 2(aq)的途径变得活跃,尽管在两条途径之间的速率常数降低了107倍,但仍有助于动力学。1 H和13 C NMR光谱作为在25.0°C下pH值降低(HCl浓度增加)的函数进行了评估,以确认DMEDA中的质子化事件。还进行了相应DMEDA氮部分装料的计算,以支持NMR质子化研究。将DMEDA动力学常数与哌嗪(PZ)的相应数据进行比较后发现,尽管DMEDA的碱度较大,但CO 2可以增强PZ的动力学性能,使其具有优越的动力学性能。在DMEDA中未观察到(aq)高于其预期的布朗斯台德反应性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号