当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Silylative Pinacol Coupling Catalyzed by Nitrogen-Doped Carbon-Encapsulated Nickel/Cobalt Nanoparticles: Evidence for a Silyl Radical Pathway
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acscatal.7b02788 Søren Kramer 1 , Fatima Hejjo 1 , Kristoffer H. Rasmussen 1 , Søren Kegnæs 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-12-27 00:00:00 , DOI: 10.1021/acscatal.7b02788 Søren Kramer 1 , Fatima Hejjo 1 , Kristoffer H. Rasmussen 1 , Søren Kegnæs 1
Affiliation
|
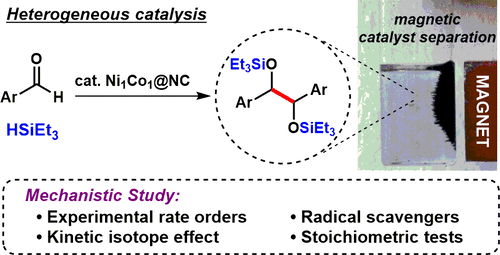
The silylative pinacol coupling of arylaldehydes catalyzed by an easily accessible, heterogeneous base-metal catalyst is demonstrated. Instead of using the classical combination of catalyst, stoichiometric metal reductants, and chlorosilanes, the developed reaction only requires the use of a catalyst and a hydrosilane, which serves as both reductant and silylating agent. A rare mechanistic investigation in this field focusing on the organic reactants was undertaken using various techniques including experimental rate orders, kinetic isotope effect, radical scavengers, and stoichiometric tests. The obtained results provided evidence for a reaction mechanism which is different from the classical pinacol coupling pathway. We propose that the heterogeneous catalyst facilitates easy access to silyl radicals, thereby circumventing the usual need for explosive initiators to access these species. In addition, leaching tests and recycling of the catalyst were performed, clearly supporting the heterogeneous nature of the catalyst.
中文翻译:

氮掺杂碳包裹的镍/钴纳米颗粒催化的甲硅烷基萘酚偶联:甲硅烷基自由基途径的证据。
结果表明,芳烃的芳基甲硅烷基化频哪醇偶联反应是通过易于获得的多相贱金属催化剂进行催化的。代替使用催化剂,化学计量的金属还原剂和氯硅烷的经典组合,开发的反应仅需要使用催化剂和氢化硅烷,后者既充当还原剂又是甲硅烷基化剂。使用各种技术,包括实验速率顺序,动力学同位素效应,自由基清除剂和化学计量测试,对有机反应物进行了罕见的机械研究。所得结果为不同于经典频哪醇偶联途径的反应机理提供了证据。我们建议,非均相催化剂有助于轻松获得甲硅烷基自由基,从而避免了通常需要使用爆炸引发剂来进入这些物质的需求。此外,还进行了催化剂的浸出试验和回收利用,清楚地证明了催化剂的非均质性。
更新日期:2017-12-27
中文翻译:

氮掺杂碳包裹的镍/钴纳米颗粒催化的甲硅烷基萘酚偶联:甲硅烷基自由基途径的证据。
结果表明,芳烃的芳基甲硅烷基化频哪醇偶联反应是通过易于获得的多相贱金属催化剂进行催化的。代替使用催化剂,化学计量的金属还原剂和氯硅烷的经典组合,开发的反应仅需要使用催化剂和氢化硅烷,后者既充当还原剂又是甲硅烷基化剂。使用各种技术,包括实验速率顺序,动力学同位素效应,自由基清除剂和化学计量测试,对有机反应物进行了罕见的机械研究。所得结果为不同于经典频哪醇偶联途径的反应机理提供了证据。我们建议,非均相催化剂有助于轻松获得甲硅烷基自由基,从而避免了通常需要使用爆炸引发剂来进入这些物质的需求。此外,还进行了催化剂的浸出试验和回收利用,清楚地证明了催化剂的非均质性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号