当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
(+)-Methyl (1R,2S)-2-{[4-(4-Chlorophenyl)-4-hydroxypiperidin-1-yl]methyl}-1-phenylcyclopropanecarboxylate [(+)-MR200] Derivatives as Potent and Selective Sigma Receptor Ligands: Stereochemistry and Pharmacological Properties
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01584 Emanuele Amata 1 , Antonio Rescifina 1 , Orazio Prezzavento 1 , Emanuela Arena 1 , Maria Dichiara 1 , Valeria Pittalà 1 , Ángeles Montilla-García 2 , Francesco Punzo 1 , Pedro Merino 3 , Enrique J. Cobos 2 , Agostino Marrazzo 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01584 Emanuele Amata 1 , Antonio Rescifina 1 , Orazio Prezzavento 1 , Emanuela Arena 1 , Maria Dichiara 1 , Valeria Pittalà 1 , Ángeles Montilla-García 2 , Francesco Punzo 1 , Pedro Merino 3 , Enrique J. Cobos 2 , Agostino Marrazzo 1
Affiliation
|
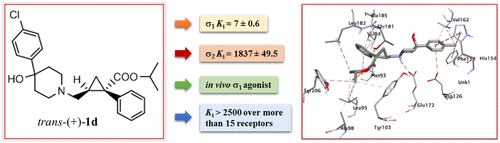
Methoxycarbonyl-1-phenyl-2-cyclopropylmethyl based derivatives cis-(+)-1a [cis-(+)-MR200], cis-(−)-1a [cis-(−)-MR201], and trans-(±)-1a [trans-(±)-MR204], have been identified as new potent sigma (σ) receptor ligands. In the present paper, novel enantiomerically pure analogues were synthesized and optimized for their σ receptor affinity and selectivity. Docking studies rationalized the results obtained in the radioligand binding assay. Absolute stereochemistry was unequivocally established by X-ray analysis of precursor trans-(+)-5a as camphorsulfonyl derivative 9. The most promising compound, trans-(+)-1d, showed remarkable selectivity over a panel of more than 15 receptors as well as good chemical and enzymatic stability in human plasma. An in vivo evaluation evidenced that trans-(+)-1d, in contrast to trans-(−)-1d, cis-(+)-1d, or cis-(−)-1d, which behave as σ1 antagonists, exhibited a σ1 agonist profile. These data clearly demonstrated that compound trans-(+)-1d, due to its σ1 agonist activity and favorable receptor selectivity and stability, provided an useful tool for the study of σ1 receptors.
中文翻译:

(+)-甲基(1 R,2 S)-2-{[4-(4-氯苯基)-4-羟基哌啶-1-基]甲基} -1-苯基环丙烷羧酸酯[(+)-MR200]衍生物和选择性西格玛受体配体:立体化学和药理特性
基于甲氧羰基-1-苯基-2-环丙基甲基的衍生物cis -(+)- 1a [ cis -(+)-MR200],cis -(-)- 1a [ cis -(-)-MR201]和反式-(± ) - 1A [反式- (±)-MR204],已被确定为新的有效西格玛(σ)受体配体。在本文中,合成了新颖的对映体纯类似物,并针对其σ受体亲和力和选择性进行了优化。对接研究使在放射性配体结合测定中获得的结果合理化。通过对前体反式-(+)- 5a作为樟脑磺酰基衍生物的X射线分析明确建立了绝对立体化学9。最有前途的化合物反式((+)- 1d)在超过15种受体上显示出显着的选择性,并在人血浆中具有良好的化学和酶稳定性。体内评价证明该反式- (+) - 1D,而相比之下,反式- ( - ) - 1D,顺式- (+) - 1D,或顺式- ( - ) - 1D,其表现为σ 1拮抗剂,表现出一个σ 1激动剂曲线。这些数据清楚地表明,化合物反式- (+) - 1D,由于其σ 1激动剂活性和良好的受体选择性和稳定性,提供了一种用于σ研究的一个有用的工具1受体。
更新日期:2017-12-20
中文翻译:

(+)-甲基(1 R,2 S)-2-{[4-(4-氯苯基)-4-羟基哌啶-1-基]甲基} -1-苯基环丙烷羧酸酯[(+)-MR200]衍生物和选择性西格玛受体配体:立体化学和药理特性
基于甲氧羰基-1-苯基-2-环丙基甲基的衍生物cis -(+)- 1a [ cis -(+)-MR200],cis -(-)- 1a [ cis -(-)-MR201]和反式-(± ) - 1A [反式- (±)-MR204],已被确定为新的有效西格玛(σ)受体配体。在本文中,合成了新颖的对映体纯类似物,并针对其σ受体亲和力和选择性进行了优化。对接研究使在放射性配体结合测定中获得的结果合理化。通过对前体反式-(+)- 5a作为樟脑磺酰基衍生物的X射线分析明确建立了绝对立体化学9。最有前途的化合物反式((+)- 1d)在超过15种受体上显示出显着的选择性,并在人血浆中具有良好的化学和酶稳定性。体内评价证明该反式- (+) - 1D,而相比之下,反式- ( - ) - 1D,顺式- (+) - 1D,或顺式- ( - ) - 1D,其表现为σ 1拮抗剂,表现出一个σ 1激动剂曲线。这些数据清楚地表明,化合物反式- (+) - 1D,由于其σ 1激动剂活性和良好的受体选择性和稳定性,提供了一种用于σ研究的一个有用的工具1受体。



























 京公网安备 11010802027423号
京公网安备 11010802027423号