当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bulk properties and transport mechanisms of a solid state antiperovskite Li-ion conductor Li3OCl: insights from first principles calculations†
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-12-08 00:00:00 , DOI: 10.1039/c7ta08780b Musheng Wu 1, 2, 3, 4, 5 , Bo Xu 1, 2, 3, 4, 5 , Xueling Lei 1, 2, 3, 4, 5 , Kelvin Huang 6, 7, 8, 9 , Chuying Ouyang 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-12-08 00:00:00 , DOI: 10.1039/c7ta08780b Musheng Wu 1, 2, 3, 4, 5 , Bo Xu 1, 2, 3, 4, 5 , Xueling Lei 1, 2, 3, 4, 5 , Kelvin Huang 6, 7, 8, 9 , Chuying Ouyang 1, 2, 3, 4, 5
Affiliation
|
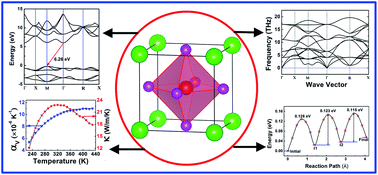
The excellent Li+ conductivity (0.85 × 10−3 S cm−1 at room temperature) and wide electrochemical stability window (>6 V) of the antiperovskite Li3OCl material make it a promising candidate electrolyte for rechargeable all-solid-state Li batteries. In this study, we systematically evaluate the electronic, mechanical, and thermodynamic properties of Li3OCl by first-principles density functional theory calculations. The defect chemistry and Li+ migration mechanisms are also discussed in the context since Li+ diffusion is strongly influenced by defects in the material. Our results show that Li3OCl is an indirect wide-band gap insulator in the equilibrium state with a band gap of ∼6.26 eV. Phonon spectral data confirm that Li3OCl is dynamically stable at its ground state. It is also revealed that Li3OCl is mechanically brittle. The bulk modulus of Li3OCl is greater than that of Li10GeP2S12, while it is comparable to that of Li0.5La0.5TiO3 and Li7La3Zr2O12. From quasi-harmonic approximation, the linear thermal expansion coefficient and thermal conductivity of the material are found to be 3.12 × 10−6 K−1 and 22.49 W m−1 K−1 at room temperature, respectively. With four types of point defect pairs in Li3OCl being considered, it is revealed that the LiCl defect pair has the lowest formation energy compared to Li2O, O substituted Cl, and Li/Li-vacancy Frenkel defect pairs. The LiCl and Frenkel defect pairs are the most important point defects responsible for the fast Li diffusion. Overall, our study provides fundamental and comprehensive insights into the bulk properties and transport mechanisms of Li3OCl for its practical application as a solid-state electrolyte in all-solid-state Li batteries.
中文翻译:

固态抗钙钛矿锂离子导体Li 3 OCl的体积性质和传输机理:从第一性原理计算得出的见解†
钙钛矿型Li 3 OCl材料具有出色的Li +电导率(室温下为0.85×10 -3 S cm -1)和宽的电化学稳定性窗口(> 6 V),使其成为可再充电全固态锂的有希望的候选电解质电池。在这项研究中,我们通过第一原理密度泛函理论计算系统地评估了Li 3 OCl的电子,机械和热力学性质。由于Li +扩散受到材料缺陷的强烈影响,因此本文还将讨论缺陷化学和Li +迁移机理。我们的结果表明,李3OCl是处于平衡状态的间接宽带隙绝缘子,带隙为〜6.26 eV。声子光谱数据证实Li 3 OCl在其基态下是动态稳定的。还显示出Li 3 OCl在机械上是脆性的。Li 3 OCl的体积模量大于Li 10 GeP 2 S 12的体积模量,而与Li 0.5 La 0.5 TiO 3和Li 7 La 3 Zr 2 O 12的体积模量相当。从准谐波近似,材料的线性热膨胀系数和导热系数在室温下分别为3.12×10 -6 K -1和22.49 W m -1 K -1。考虑到Li 3 OCl中的四种类型的点缺陷对,发现与Li 2 O,O取代的Cl和Li / Li空位Frenkel缺陷对相比,LiCl缺陷对具有最低的形成能。LiCl和Frenkel缺陷对是导致Li快速扩散的最重要点缺陷。总体而言,我们的研究为Li 3的整体性质和传输机理提供了基础和全面的见解。OCl在全固态锂电池中作为固态电解质的实际应用。
更新日期:2017-12-08
中文翻译:

固态抗钙钛矿锂离子导体Li 3 OCl的体积性质和传输机理:从第一性原理计算得出的见解†
钙钛矿型Li 3 OCl材料具有出色的Li +电导率(室温下为0.85×10 -3 S cm -1)和宽的电化学稳定性窗口(> 6 V),使其成为可再充电全固态锂的有希望的候选电解质电池。在这项研究中,我们通过第一原理密度泛函理论计算系统地评估了Li 3 OCl的电子,机械和热力学性质。由于Li +扩散受到材料缺陷的强烈影响,因此本文还将讨论缺陷化学和Li +迁移机理。我们的结果表明,李3OCl是处于平衡状态的间接宽带隙绝缘子,带隙为〜6.26 eV。声子光谱数据证实Li 3 OCl在其基态下是动态稳定的。还显示出Li 3 OCl在机械上是脆性的。Li 3 OCl的体积模量大于Li 10 GeP 2 S 12的体积模量,而与Li 0.5 La 0.5 TiO 3和Li 7 La 3 Zr 2 O 12的体积模量相当。从准谐波近似,材料的线性热膨胀系数和导热系数在室温下分别为3.12×10 -6 K -1和22.49 W m -1 K -1。考虑到Li 3 OCl中的四种类型的点缺陷对,发现与Li 2 O,O取代的Cl和Li / Li空位Frenkel缺陷对相比,LiCl缺陷对具有最低的形成能。LiCl和Frenkel缺陷对是导致Li快速扩散的最重要点缺陷。总体而言,我们的研究为Li 3的整体性质和传输机理提供了基础和全面的见解。OCl在全固态锂电池中作为固态电解质的实际应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号