当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Latent Warheads for Targeted Cancer Therapy: Design and Synthesis of pro-Pyrrolobenzodiazepines and Conjugates
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-12-06 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00476 Iontcho R. Vlahov 1 , Longwu Qi 1 , Paul J. Kleindl 1 , Hari K. Santhapuram 1 , Albert Felten 1 , Garth L. Parham 1 , Kevin Wang 1 , Fei You 1 , Jeremy F. Vaughn 1 , Spencer J. Hahn 1 , Hanna F. Klein 1 , Marilynn Vetzel 1 , Joseph A. Reddy 1 , Melissa Nelson 1 , Jeff Nicoson 1 , Christopher P. Leamon 1
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-12-06 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00476 Iontcho R. Vlahov 1 , Longwu Qi 1 , Paul J. Kleindl 1 , Hari K. Santhapuram 1 , Albert Felten 1 , Garth L. Parham 1 , Kevin Wang 1 , Fei You 1 , Jeremy F. Vaughn 1 , Spencer J. Hahn 1 , Hanna F. Klein 1 , Marilynn Vetzel 1 , Joseph A. Reddy 1 , Melissa Nelson 1 , Jeff Nicoson 1 , Christopher P. Leamon 1
Affiliation
|
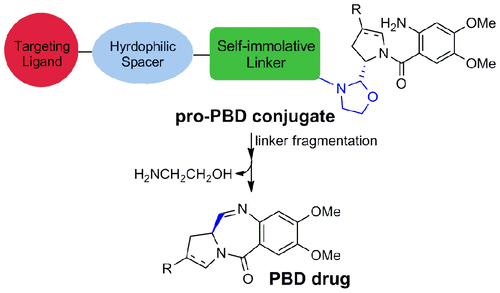
Pyrrolobenzodiazepines (PBDs) and their dimers (bis-PBDs) have emerged as some of the most potent chemotherapeutic compounds, and are currently under development as novel payloads in antibody–drug conjugates (ADCs). However, when used as stand-alone therapeutics or as warheads for small molecule drug conjugates (SMDCs), dose-limiting toxicities are often observed. As an elegant solution to this inherent problem, we designed diazepine-ring-opened conjugated prodrugs lacking the imine moiety. Once the prodrug (pro-PBD) conjugate enters a targeted cell, cleavage of the linker system triggers the generation of a reactive intermediate possessing an aldehyde and aromatic amine. An intramolecular ring-closing reaction subsequently takes place as the aromatic amine adds to the aldehyde with the loss of water to give the imine and, as a result, the diazepine ring. In our pro-PBDs, we mask the aldehyde as a hydrolytically sensitive oxazolidine moiety which in turn is a part of a reductively labile self-immolative linker system. To prove the range of applications for this new class of latent DNA-alkylators, we designed and synthesized several novel latent warheads: pro-PBD dimers and hybrids of pro-PBD with other sequence-selective DNA minor groove binders. Preliminary preclinical pharmacology studies showed excellent biological activity and specificity.
中文翻译:

靶向癌症治疗的潜在战斗部:吡咯并苯二氮杂卓和缀合物的设计与合成
吡咯并二氮杂卓(PBD)及其二聚体(bis-PBD)已作为一些最有效的化学治疗化合物出现,目前正在开发为抗体-药物偶联物(ADC)中的新型有效负载。但是,当用作独立疗法或用作小分子药物偶联物(SMDC)的战斗部时,经常会观察到剂量限制的毒性。为了解决这个固有问题,我们设计了缺少亚胺部分的二氮杂苯环开环的共轭前药。一旦前药(pro-PBD)缀合物进入目标细胞,连接系统的裂解就会触发具有醛和芳香胺的反应性中间体的生成。随后发生分子内的闭环反应,这是因为芳族胺加到醛中而损失了水而得到亚胺,结果,二氮杂ring环。在我们的前PBD中,我们将醛掩盖为水解敏感的恶唑烷部分,而后者又是还原不稳定的自焚连接系统的一部分。为了证明这种新型的潜在DNA-烷基化剂的应用范围,我们设计并合成了几种新型的潜在弹头:pro-PBD二聚体和pro-PBD与其他序列选择性DNA小沟结合剂的混合物。初步的临床前药理研究显示出极好的生物学活性和特异性。pro-PBD二聚体和pro-PBD与其他序列选择性DNA小沟结合剂的杂种。初步的临床前药理研究显示出极好的生物学活性和特异性。pro-PBD二聚体和pro-PBD与其他序列选择性DNA小沟结合剂的杂种。初步的临床前药理研究显示出极好的生物学活性和特异性。
更新日期:2017-12-06
中文翻译:

靶向癌症治疗的潜在战斗部:吡咯并苯二氮杂卓和缀合物的设计与合成
吡咯并二氮杂卓(PBD)及其二聚体(bis-PBD)已作为一些最有效的化学治疗化合物出现,目前正在开发为抗体-药物偶联物(ADC)中的新型有效负载。但是,当用作独立疗法或用作小分子药物偶联物(SMDC)的战斗部时,经常会观察到剂量限制的毒性。为了解决这个固有问题,我们设计了缺少亚胺部分的二氮杂苯环开环的共轭前药。一旦前药(pro-PBD)缀合物进入目标细胞,连接系统的裂解就会触发具有醛和芳香胺的反应性中间体的生成。随后发生分子内的闭环反应,这是因为芳族胺加到醛中而损失了水而得到亚胺,结果,二氮杂ring环。在我们的前PBD中,我们将醛掩盖为水解敏感的恶唑烷部分,而后者又是还原不稳定的自焚连接系统的一部分。为了证明这种新型的潜在DNA-烷基化剂的应用范围,我们设计并合成了几种新型的潜在弹头:pro-PBD二聚体和pro-PBD与其他序列选择性DNA小沟结合剂的混合物。初步的临床前药理研究显示出极好的生物学活性和特异性。pro-PBD二聚体和pro-PBD与其他序列选择性DNA小沟结合剂的杂种。初步的临床前药理研究显示出极好的生物学活性和特异性。pro-PBD二聚体和pro-PBD与其他序列选择性DNA小沟结合剂的杂种。初步的临床前药理研究显示出极好的生物学活性和特异性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号