当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrophobic Ion Pairing of Peptide Antibiotics for Processing into Controlled Release Nanocarrier Formulations
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00824 Hoang D. Lu 1 , Paradorn Rummaneethorn 1 , Kurt D. Ristroph 1 , Robert K. Prud’homme 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00824 Hoang D. Lu 1 , Paradorn Rummaneethorn 1 , Kurt D. Ristroph 1 , Robert K. Prud’homme 1
Affiliation
|
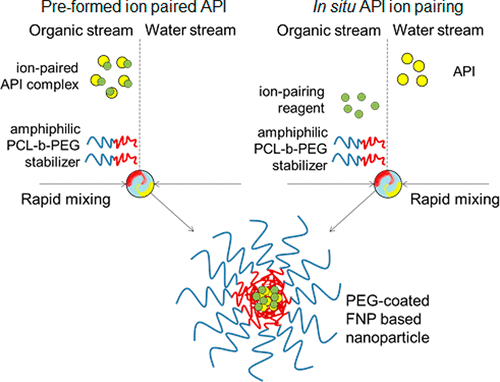
Nanoprecipitation of active pharmaceutical ingredients (APIs) to form nanocarriers (NCs) is an attractive method of producing formulations with improved stability and biological efficacies. However, nanoprecipitation techniques have not been demonstrated for highly soluble peptide therapeutics. We here present a model and technique to encapsulate highly water-soluble biologic APIs by manipulating API salt forms. APIs are ion paired with hydrophobic counterions to produce new API salts that exhibit altered solubilities suitable for nanoprecipitation processing. The governing rules of ion pair identity and processing conditions required for successful encapsulation are experimentally determined and assessed with theoretical models. Successful NC formation for the antibiotic polymyxin B requires hydrophobicity of the ion pair acid to be greater than logP = 2 for strong acids and greater than logP = 8 for weak acids. Oleic acid with a logP = 8, and pKa = 5, appears to be a prime candidate as an ion pair agent since it is biocompatible and forms excellent ion pair complexes. NC formation from preformed, organic soluble ion pairs is compared to in situ ion pairs where NCs are made in a single precipitation step. NC properties, such as stability and release rates, can be tuned by varying ion pair molecular structure and ion pair-to-API molar ratios. For polymyxin B, NCs ≈ 100–200 nm in size, displaying API release rates over 3 days, were produced. This work demonstrates a new approach that enables the formation of nanoparticles from previously intractable compounds.
中文翻译:

肽抗生素的疏水离子配对,用于加工成控释纳米载体制剂
活性药物成分(API)的纳米沉淀以形成纳米载体(NCs)是生产具有改善的稳定性和生物功效的制剂的一种有吸引力的方法。但是,尚未证明纳米沉淀技术可用于高度可溶性的肽治疗剂。我们在这里提出了一种通过操纵API盐形式来封装高度水溶性生物API的模型和技术。API与疏水性抗衡离子进行离子配对以产生新的API盐,这些API盐显示出改变的溶解度,适用于纳米沉淀工艺。成功地封装所需的离子对身份和处理条件的控制规则是通过实验确定的,并通过理论模型进行了评估。抗生素多粘菌素B的成功NC形成要求离子对酸的疏水性对于强酸大于logP = 2,对于弱酸大于logP = 8。logP = 8且p的油酸K a = 5似乎是作为离子对试剂的主要候选物,因为它具有生物相容性并形成优异的离子对络合物。将预先形成的有机可溶离子对形成的NC与在单个沉淀步骤中制备NC的原位离子对进行比较。可以通过改变离子对分子结构和离子对与API的摩尔比来调节NC性能,例如稳定性和释放速率。对于多粘菌素B,产生的NCs≈100–200 nm,显示3天内API释放速率。这项工作演示了一种新方法,该方法能够使以前难以处理的化合物形成纳米颗粒。
更新日期:2017-12-05
中文翻译:

肽抗生素的疏水离子配对,用于加工成控释纳米载体制剂
活性药物成分(API)的纳米沉淀以形成纳米载体(NCs)是生产具有改善的稳定性和生物功效的制剂的一种有吸引力的方法。但是,尚未证明纳米沉淀技术可用于高度可溶性的肽治疗剂。我们在这里提出了一种通过操纵API盐形式来封装高度水溶性生物API的模型和技术。API与疏水性抗衡离子进行离子配对以产生新的API盐,这些API盐显示出改变的溶解度,适用于纳米沉淀工艺。成功地封装所需的离子对身份和处理条件的控制规则是通过实验确定的,并通过理论模型进行了评估。抗生素多粘菌素B的成功NC形成要求离子对酸的疏水性对于强酸大于logP = 2,对于弱酸大于logP = 8。logP = 8且p的油酸K a = 5似乎是作为离子对试剂的主要候选物,因为它具有生物相容性并形成优异的离子对络合物。将预先形成的有机可溶离子对形成的NC与在单个沉淀步骤中制备NC的原位离子对进行比较。可以通过改变离子对分子结构和离子对与API的摩尔比来调节NC性能,例如稳定性和释放速率。对于多粘菌素B,产生的NCs≈100–200 nm,显示3天内API释放速率。这项工作演示了一种新方法,该方法能够使以前难以处理的化合物形成纳米颗粒。


























 京公网安备 11010802027423号
京公网安备 11010802027423号