当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding of 12-Crown-4 with Alzheimer’s Aβ40 and Aβ42 Monomers and Its Effect on Their Conformation: Insight from Molecular Dynamics Simulations
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-12-15 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00966 Nikhil Agrawal 1 , Adam A. Skelton 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-12-15 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00966 Nikhil Agrawal 1 , Adam A. Skelton 1
Affiliation
|
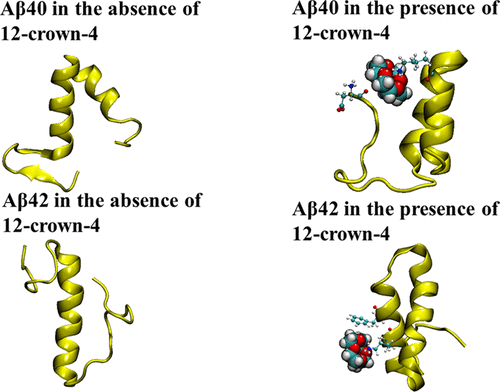
Alzheimer’s disease is the most common form of dementia and is considered to be caused by the conformational change of Aβ monomers, from their native monomeric states, to form Aβ oligomers/aggregates in the brain. Turn formation in Aβ monomer has been suggested to be the nucleation step for Aβ misfolding. In the present work, we have performed a series of all-atom molecular dynamics simulations, a total time of 11.4 μs, to elucidate factor that contributes for early stage misfolding of Aβ40 and Aβ42 monomers and reveals the binding modes of 12-crown-4 on Aβ40 and Aβ42 monomer and effect of its binding on structural stability. Our simulation data revealed that the region around Val24-Lys28 is most prevalent for turn formation and a gain of water molecules around Lys28 side chains occurs at the same time as a significant gain in conformational entropy of the side chain. The initiation steps lead a greater number of water molecules available and enhancement of the conformational entropy of the backbone atoms; this leads to greater probability of breaking Lys28 backbone intrapeptide H-bonds, and consequently turns formation. Simulations of Aβ40 and Aβ42 monomers with 12-crown-4 showed that the molecule is highly specific toward positively charged Lys16, Lys28 residues, and N-terminal Asp1. Lys16 and Asp1 have been previously reported to make Aβ peptide toxic. Our secondary structure analysis revealed that in the absence of 12-crown-4 there was a β-sheet formed in the Aβ40 peptide. In case of Aβ42 monomer, in the absence of 12-crown-4, we observed that the second helix region converted into a coil and turn; however, in the presence of 12-crown-4 it remained stable. Observed pharmacophore features of, 12-crown-4 will not only help in designing new candidate drug molecules, which are specific to Aβ peptides but could also be used to design new imaging probe molecules, which could be used for labeling Aβ peptide
中文翻译:

12-Crown-4与阿尔茨海默氏症Aβ40和Aβ42单体的结合及其对构象的影响:分子动力学模拟的启示
阿尔茨海默氏病是最常见的痴呆形式,被认为是由Aβ单体的构象变化引起的,从其天然单体状态转变为大脑中的Aβ寡聚体/聚集体。有人提出在Aβ单体中形成转弯是Aβ错折叠的成核步骤。在目前的工作中,我们进行了一系列全原子分子动力学模拟,总时间为11.4μs,以阐明有助于Aβ40和Aβ42单体早期错折叠并揭示12冠4结合模式的因素。对Aβ40和Aβ42单体的影响及其结合对结构稳定性的影响。我们的模拟数据显示,Val24-Lys28周围的区域最常形成转弯,Lys28侧链周围的水分子增加与侧链构象熵的显着增加同时发生。引发步骤导致更多的水分子可用并增强了骨架原子的构象熵。这导致破坏Lys28骨架内肽H键的更大可能性,并因此导致形成。用12-crown-4模拟Aβ40和Aβ42单体表明,该分子对带正电的Lys16,Lys28残基和N末端Asp1具有高度特异性。先前已报道Lys16和Asp1使Aβ肽具有毒性。我们的二级结构分析表明,在不存在12冠4的情况下,Aβ40肽中形成了一个β-折叠。在Aβ42单体的情况下,在不存在12冠4的情况下,我们观察到第二个螺旋区域转化为线圈并转向;但是,在有12冠4的情况下,它保持稳定。观察到的12-crown-4的药效团特征不仅有助于设计针对Aβ肽的新候选药物分子,而且还可以用于设计可用于标记Aβ肽的新的成像探针分子。
更新日期:2017-12-15
中文翻译:

12-Crown-4与阿尔茨海默氏症Aβ40和Aβ42单体的结合及其对构象的影响:分子动力学模拟的启示
阿尔茨海默氏病是最常见的痴呆形式,被认为是由Aβ单体的构象变化引起的,从其天然单体状态转变为大脑中的Aβ寡聚体/聚集体。有人提出在Aβ单体中形成转弯是Aβ错折叠的成核步骤。在目前的工作中,我们进行了一系列全原子分子动力学模拟,总时间为11.4μs,以阐明有助于Aβ40和Aβ42单体早期错折叠并揭示12冠4结合模式的因素。对Aβ40和Aβ42单体的影响及其结合对结构稳定性的影响。我们的模拟数据显示,Val24-Lys28周围的区域最常形成转弯,Lys28侧链周围的水分子增加与侧链构象熵的显着增加同时发生。引发步骤导致更多的水分子可用并增强了骨架原子的构象熵。这导致破坏Lys28骨架内肽H键的更大可能性,并因此导致形成。用12-crown-4模拟Aβ40和Aβ42单体表明,该分子对带正电的Lys16,Lys28残基和N末端Asp1具有高度特异性。先前已报道Lys16和Asp1使Aβ肽具有毒性。我们的二级结构分析表明,在不存在12冠4的情况下,Aβ40肽中形成了一个β-折叠。在Aβ42单体的情况下,在不存在12冠4的情况下,我们观察到第二个螺旋区域转化为线圈并转向;但是,在有12冠4的情况下,它保持稳定。观察到的12-crown-4的药效团特征不仅有助于设计针对Aβ肽的新候选药物分子,而且还可以用于设计可用于标记Aβ肽的新的成像探针分子。


























 京公网安备 11010802027423号
京公网安备 11010802027423号