当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
3-Year Clinical Outcomes With Everolimus-Eluting Bioresorbable Coronary Scaffolds
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2017-12-01 , DOI: 10.1016/j.jacc.2017.10.010 Dean J Kereiakes 1 , Stephen G Ellis 2 , Christopher Metzger 3 , Ronald P Caputo 4 , David G Rizik 5 , Paul S Teirstein 6 , Marc R Litt 7 , Annapoorna Kini 8 , Ameer Kabour 9 , Steven O Marx 10 , Jeffrey J Popma 11 , Robert McGreevy 12 , Zhen Zhang 12 , Charles Simonton 12 , Gregg W Stone 10 ,
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2017-12-01 , DOI: 10.1016/j.jacc.2017.10.010 Dean J Kereiakes 1 , Stephen G Ellis 2 , Christopher Metzger 3 , Ronald P Caputo 4 , David G Rizik 5 , Paul S Teirstein 6 , Marc R Litt 7 , Annapoorna Kini 8 , Ameer Kabour 9 , Steven O Marx 10 , Jeffrey J Popma 11 , Robert McGreevy 12 , Zhen Zhang 12 , Charles Simonton 12 , Gregg W Stone 10 ,
Affiliation
|
BACKGROUND
The Absorb everolimus-eluting poly-L-lactic acid-based bioresorbable vascular scaffold (BVS) provides early drug delivery and mechanical support functions similar to metallic drug-eluting stents (DES), followed by complete bioresorption in approximately 3 years with recovery of vascular structure and function. The ABSORB III trial demonstrated noninferior rates of target lesion failure (cardiac death, target vessel myocardial infarction [TVMI], or ischemia-driven target lesion revascularization) at 1 year in 2,008 patients with coronary artery disease randomized to BVS versus cobalt-chromium everolimus-eluting stents (EES). OBJECTIVES
This study sought to assess clinical outcomes through 3 years following BVS implantation. METHODS
Clinical outcomes from the ABSORB III trial were analyzed by randomized treatment assignment cumulative through 3 years, and between 1 and 3 years. RESULTS
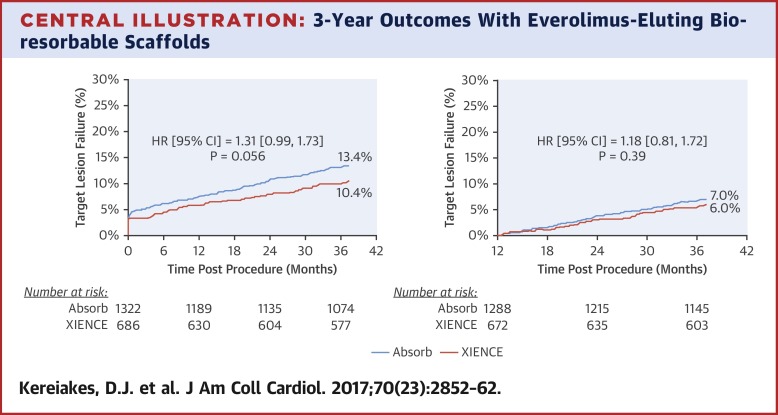
The primary composite endpoint of target lesion failure through 3 years occurred in 13.4% of BVS patients and 10.4% of EES patients (p = 0.06), and between 1 and 3 years in 7.0% versus 6.0% of patients, respectively (p = 0.39). TVMI through 3 years was increased with BVS (8.6% vs. 5.9%; p = 0.03), as was device thrombosis (2.3% vs. 0.7%; p = 0.01). In BVS-assigned patients, treatment of very small vessels (those with quantitatively determined reference vessel diameter <2.25 mm) was an independent predictor of 3-year TLF and scaffold thrombosis. CONCLUSIONS
In the ABSORB III trial, 3-year adverse event rates were higher with BVS than EES, particularly TVMI and device thrombosis. Longer-term clinical follow-up is required to determine whether bioresorption of the polymeric scaffold will influence patient prognosis. (ABSORB III Randomized Controlled Trial [RCT] [ABSORB-III]; NCT01751906).
中文翻译:

使用依维莫司洗脱生物可吸收冠状动脉支架的 3 年临床结果
背景 Absorb依维莫司洗脱聚-L-乳酸基生物可吸收血管支架(BVS)提供类似于金属药物洗脱支架(DES)的早期药物输送和机械支撑功能,随后在大约3年内完全生物吸收,恢复血管结构和功能。ABSORB III 试验表明,2,008 名冠状动脉疾病患者随机接受 BVS 与钴铬依维莫司治疗,1 年时靶病变失败率(心脏死亡、靶血管心肌梗死 [TVMI] 或缺血驱动的靶病变血运重建)具有非劣效性。洗脱支架 (EES)。目的 本研究旨在评估 BVS 植入后 3 年内的临床结果。方法 ABSORB III 试验的临床结果通过 3 年和 1 至 3 年累积的随机治疗分配进行分析。结果 13.4% 的 BVS 患者和 10.4% 的 EES 患者 (p = 0.06) 和 1 至 3 年之间的 3 年目标病变失败的主要复合终点分别发生在 7.0% 和 6.0% 的患者中 (p = 0.39)。BVS 增加了 3 年的 TVMI(8.6% 对 5.9%;p = 0.03),以及装置血栓形成(2.3% 对 0.7%;p = 0.01)。在 BVS 分配的患者中,治疗非常小的血管(那些定量确定的参考血管直径 <2.25 毫米)是 3 年 TLF 和支架血栓形成的独立预测因子。结论 在 ABSORB III 试验中,BVS 的 3 年不良事件发生率高于 EES,尤其是 TVMI 和器械血栓形成。需要更长期的临床随访来确定聚合物支架的生物再吸收是否会影响患者的预后。(ABSORB III 随机对照试验 [RCT] [ABSORB-III];NCT01751906)。
更新日期:2017-12-01
中文翻译:

使用依维莫司洗脱生物可吸收冠状动脉支架的 3 年临床结果
背景 Absorb依维莫司洗脱聚-L-乳酸基生物可吸收血管支架(BVS)提供类似于金属药物洗脱支架(DES)的早期药物输送和机械支撑功能,随后在大约3年内完全生物吸收,恢复血管结构和功能。ABSORB III 试验表明,2,008 名冠状动脉疾病患者随机接受 BVS 与钴铬依维莫司治疗,1 年时靶病变失败率(心脏死亡、靶血管心肌梗死 [TVMI] 或缺血驱动的靶病变血运重建)具有非劣效性。洗脱支架 (EES)。目的 本研究旨在评估 BVS 植入后 3 年内的临床结果。方法 ABSORB III 试验的临床结果通过 3 年和 1 至 3 年累积的随机治疗分配进行分析。结果 13.4% 的 BVS 患者和 10.4% 的 EES 患者 (p = 0.06) 和 1 至 3 年之间的 3 年目标病变失败的主要复合终点分别发生在 7.0% 和 6.0% 的患者中 (p = 0.39)。BVS 增加了 3 年的 TVMI(8.6% 对 5.9%;p = 0.03),以及装置血栓形成(2.3% 对 0.7%;p = 0.01)。在 BVS 分配的患者中,治疗非常小的血管(那些定量确定的参考血管直径 <2.25 毫米)是 3 年 TLF 和支架血栓形成的独立预测因子。结论 在 ABSORB III 试验中,BVS 的 3 年不良事件发生率高于 EES,尤其是 TVMI 和器械血栓形成。需要更长期的临床随访来确定聚合物支架的生物再吸收是否会影响患者的预后。(ABSORB III 随机对照试验 [RCT] [ABSORB-III];NCT01751906)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号