当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Densities and Viscosities for Binary Liquid Mixtures of Butan-1-ol + Propane-1,2-diol, + Butane-1,2-diol and 2-Methylpropan-1-ol + Propane-1,2-diol, + Butane-1,2-diol from 298.15 to 333.15 K at 0.1 MPa
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1021/acs.jced.7b00621 José J. Cano-Gómez 1 , Gustavo A. Iglesias-Silva 2 , Luis D. Cortez-Sánchez 1 , María T. Castillo-Escobedo 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1021/acs.jced.7b00621 José J. Cano-Gómez 1 , Gustavo A. Iglesias-Silva 2 , Luis D. Cortez-Sánchez 1 , María T. Castillo-Escobedo 1
Affiliation
|
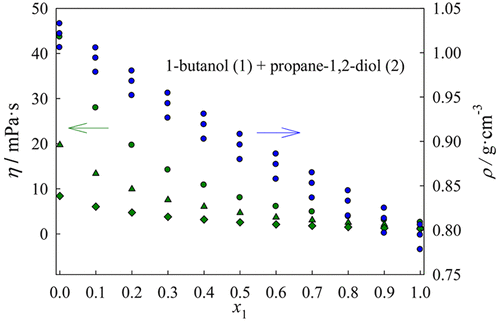
This paper presents densities and viscosities of binary mixtures of butan-1-ol (or 2-methylpropan-1-ol) with propane-1,2-diol, and butane-1,2-diol from 298.15 to 333.15 K at 0.1 MPa over the entire concentration range. A vibrating tube densimeter provides the densities while a glass capillary viscometer provides the efflux time which is related with the kinematic viscosity. Experimental densities and viscosities of the pure components agree with data reported in the literature within an average absolute percentage deviation of 0.03% and 1.16%, respectively. The excess molar volumes and viscosity deviation calculated from experimental data present negative deviations from ideality in the entire temperature range. The Redlich–Kister equation is used to represent the composition behavior of excess molar volumes and the viscosity deviations. The Nava-Rios equation correlates our kinematic viscosity data within an overall average absolute percentage deviation of 1.085% while the McAllister equation correlates the kinematic viscosity within 1.541%.
中文翻译:

丁-1-醇+丙烷-1,2-二醇,+丁烷-1,2-二醇和2-甲基丙-1-醇+丙烷-1,2-二醇,+丁烷-的二元液体混合物的密度和粘度1,2-二醇从298.15至333.15 K在0.1 MPa下
本文介绍了0.1 MPa下298.15至333.15 K范围内丁烷1-醇(或2-甲基丙-1-醇)与丙烷1,2-二醇和丁烷1,1,2-二醇的二元混合物的密度和粘度在整个浓度范围内。振动管密度计提供密度,而玻璃毛细管粘度计提供与运动粘度有关的流出时间。纯组分的实验密度和粘度分别与文献报道的数据相符,平均绝对百分偏差分别为0.03%和1.16%。根据实验数据计算出的过量摩尔体积和粘度偏差在整个温度范围内均表现出与理想状态的负偏差。Redlich–Kister方程用于表示过量摩尔体积和粘度偏差的组成行为。
更新日期:2017-12-05
中文翻译:

丁-1-醇+丙烷-1,2-二醇,+丁烷-1,2-二醇和2-甲基丙-1-醇+丙烷-1,2-二醇,+丁烷-的二元液体混合物的密度和粘度1,2-二醇从298.15至333.15 K在0.1 MPa下
本文介绍了0.1 MPa下298.15至333.15 K范围内丁烷1-醇(或2-甲基丙-1-醇)与丙烷1,2-二醇和丁烷1,1,2-二醇的二元混合物的密度和粘度在整个浓度范围内。振动管密度计提供密度,而玻璃毛细管粘度计提供与运动粘度有关的流出时间。纯组分的实验密度和粘度分别与文献报道的数据相符,平均绝对百分偏差分别为0.03%和1.16%。根据实验数据计算出的过量摩尔体积和粘度偏差在整个温度范围内均表现出与理想状态的负偏差。Redlich–Kister方程用于表示过量摩尔体积和粘度偏差的组成行为。



























 京公网安备 11010802027423号
京公网安备 11010802027423号