当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility of NaHCO3 and NH4HCO3 in the Relevant Media and Prediction of High-Pressure Phase Equilibria for the NH3–CO2–NaCl–H2O System
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1021/acs.jced.7b00790 Juan Zhou 1 , Yan Zeng 2 , George P. Demopoulos 2 , Chunxi Li 1 , Zhibao Li 3
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1021/acs.jced.7b00790 Juan Zhou 1 , Yan Zeng 2 , George P. Demopoulos 2 , Chunxi Li 1 , Zhibao Li 3
Affiliation
|
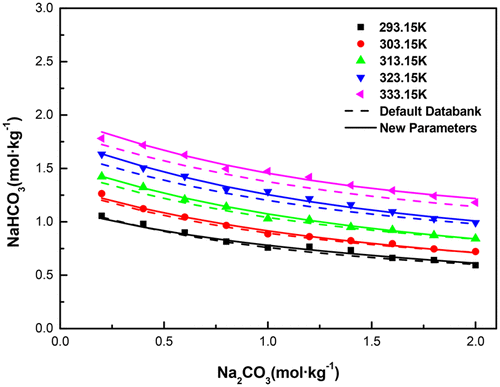
The solubility of NaHCO3 was determined in the NH4+–Na+–CO32––HCO3––Cl––H2O system with the concentration up to 6 mol·kg–1 by a dynamic method from 283.15 to 333.15 K. It was found that the solubility of NaHCO3 decreases with increasing concentration of Na2CO3, NH4HCO3, or NaCl. A speciation-based model was developed with mixed-solvent electrolyte interaction parameters newly obtained by regressing both the experimental and literature solubility data. The average relative deviations are less than 5.0%. The new model is able to predict the solubility of NaHCO3 at high pressures up to 6.0 MPa for the NH3–CO2–NaCl–H2O system at 313.15 and 323.15 K. This work can provide a theoretical basis for producing Na2CO3 with a better crystal form and higher productivity in the carbonation process.
中文翻译:

NaHCO 3和NH 4 HCO 3在相关介质中的溶解度以及NH 3 -CO 2 -NaCl-H 2 O系统高压相平衡的预测
碳酸氢钠的溶解度3是在NH确定4 + -Na + -CO 3 2- -HCO 3 - -Cl - -H 2 O,其浓度可达系统6摩尔公斤· -1通过动态方法从283.15到333.15K。发现NaHCO 3的溶解度随Na 2 CO 3,NH 4 HCO 3浓度的增加而降低或NaCl。通过回归实验和文献溶解度数据,开发了一种基于形态的模型,该模型具有新获得的混合溶剂电解质相互作用参数。平均相对偏差小于5.0%。对于在313.15和323.15 K下的NH 3 –CO 2 –NaCl–H 2 O系统,新模型能够预测在高达6.0 MPa的高压下NaHCO 3的溶解度。这项工作可以为生产Na 2提供理论依据。在碳化过程中,具有更好的晶体形式和更高的生产率的CO 3。
更新日期:2017-12-04
中文翻译:

NaHCO 3和NH 4 HCO 3在相关介质中的溶解度以及NH 3 -CO 2 -NaCl-H 2 O系统高压相平衡的预测
碳酸氢钠的溶解度3是在NH确定4 + -Na + -CO 3 2- -HCO 3 - -Cl - -H 2 O,其浓度可达系统6摩尔公斤· -1通过动态方法从283.15到333.15K。发现NaHCO 3的溶解度随Na 2 CO 3,NH 4 HCO 3浓度的增加而降低或NaCl。通过回归实验和文献溶解度数据,开发了一种基于形态的模型,该模型具有新获得的混合溶剂电解质相互作用参数。平均相对偏差小于5.0%。对于在313.15和323.15 K下的NH 3 –CO 2 –NaCl–H 2 O系统,新模型能够预测在高达6.0 MPa的高压下NaHCO 3的溶解度。这项工作可以为生产Na 2提供理论依据。在碳化过程中,具有更好的晶体形式和更高的生产率的CO 3。


























 京公网安备 11010802027423号
京公网安备 11010802027423号