Molecular Catalysis ( IF 4.6 ) Pub Date : 2017-12-01 , DOI: 10.1016/j.mcat.2017.11.034 Xiaobin Hao , Riguang Zhang , Leilei He , Zaixing Huang , Baojun Wang
|
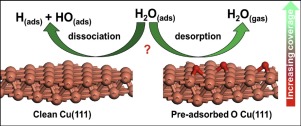
Density functional theory calculations have been employed to investigate the adsorption and dissociation of H2O, as well as the aggregation of H2O over the clean and pre-adsorbed oxygen Cu(111) surface at different coverage. As the coverage increases, the adsorption ability of H2O, OH, H and O species becomes gradually weaker, H2O dissociation becomes more unfavorable on both the clean and pre-adsorbed oxygen Cu(111) surface. H2O desorption is more favorable than its dissociation on the clean Cu(111) surface at the different coverage due to the weakly physical interaction between H2O molecule and Cu surface. However, on the pre-adsorbed oxygen Cu(111) surface, the adsorbed oxygen atom promotes H2O dissociation at different coverage, and H2O prefers to be dissociated instead of its desorption at the coverage below 0.50 ML. For H2O aggregation over Cu(111) surface, as the number of H2O molecule increases, the aggregation becomes easier due to the stronger hydrogen-bond interaction among different adsorbed H2O molecules, while the interaction between (H2O)n and Cu surface becomes weaker.
中文翻译:

在清洁和预先吸附的氧气Cu(111)表面上H 2 O的覆盖率依赖性吸附,解离和聚集:DFT研究
密度泛函理论计算已被用来研究H 2 O的吸附和解离,以及在不同覆盖率下在干净和预吸附的氧Cu(111)表面上H 2 O的聚集。随着覆盖率的增加,H 2 O,OH,H和O物种的吸附能力逐渐变弱,在干净的和预吸附的氧Cu(111)表面上H 2 O的离解变得更加不利。ħ 2 ö解吸比其清洁的Cu(111)在不同的覆盖表面上的解离更有利的,由于与H之间的弱物理相互作用2O分子和Cu表面。然而,在预吸附的氧Cu(111)表面上,吸附的氧原子在不同的覆盖率下促进H 2 O的离解,并且H 2 O倾向于离解而不是在低于0.50 ML的覆盖率下解吸。对于Cu(111)表面上的H 2 O聚集,随着H 2 O分子数量的增加,由于不同吸附的H 2 O分子之间更强的氢键相互作用,而(H 2 O )n和Cu表面变弱。



























 京公网安备 11010802027423号
京公网安备 11010802027423号