当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mobilization of iron from ferritin: new steps and details
Metallomics ( IF 3.4 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1039/c7mt00284j A. La 1, 2, 3, 4 , T. Nguyen 1, 2, 3, 4 , K. Tran 1, 2, 3, 4 , E. Sauble 1, 2, 3, 4 , D. Tu 1, 2, 3, 4 , A. Gonzalez 1, 2, 3, 4 , T. Z. Kidane 1, 2, 3, 4 , C. Soriano 1, 2, 3, 4 , J. Morgan 1, 2, 3, 4 , M. Doan 1, 2, 3, 4 , K. Tran 1, 2, 3, 4 , C.-Y. Wang 4, 5, 6, 7 , M. D. Knutson 4, 5, 6, 7 , M. C. Linder 1, 2, 3, 4
Metallomics ( IF 3.4 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1039/c7mt00284j A. La 1, 2, 3, 4 , T. Nguyen 1, 2, 3, 4 , K. Tran 1, 2, 3, 4 , E. Sauble 1, 2, 3, 4 , D. Tu 1, 2, 3, 4 , A. Gonzalez 1, 2, 3, 4 , T. Z. Kidane 1, 2, 3, 4 , C. Soriano 1, 2, 3, 4 , J. Morgan 1, 2, 3, 4 , M. Doan 1, 2, 3, 4 , K. Tran 1, 2, 3, 4 , C.-Y. Wang 4, 5, 6, 7 , M. D. Knutson 4, 5, 6, 7 , M. C. Linder 1, 2, 3, 4
Affiliation
|
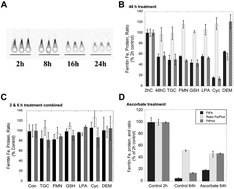
Much evidence indicates that iron stored in ferritin is mobilized through protein degradation in lysosomes, but concerns about this process have lingered, and the mechanistic details of its aspects are lacking. In the studies presented here, 59Fe-labeled ferritin was induced by preloading hepatic (HepG2) cells with radiolabeled Fe. Placing these cells in a medium containing desferrioxamine resulted in the loss of ferritin-59Fe, but adding high concentrations of reducing agents or modulating the internal GSH concentration failed to alter the rates of ferritin-59Fe release. Confocal microscopy showed that Fe deprivation increased the movement of ferritin into lysosomes and hyperaccumulation was observed when lysosomal proteolysis was inhibited. It also resulted in the rapid movement of DMT1 to lysosomes, which was inhibited by bafilomycin. Ferrihydrite crystals isolated from purified rat liver/spleen ferritin were solubilized at pH 5 and 7 by GSH, ascorbate, citrate and lysosomal fluids obtained from livers and J774a.1 macrophages. The inhibition of DMT1/Nramp2 and siRNA knockdown of Nramp1 each reduced the transfer of 59Fe from lysosomes to the cytosol; and hepatocyte-specific knockout of DMT1 in mice prevented the release of Fe from the liver responding to EPO treatment, but did not inhibit lysosomal ferritin degradation. We conclude that ferritin-Fe mobilization does not occur through changes in cellular concentrations of reducing/chelating agents but by the coordinated movement of ferritin and DMT1 to lysosomes, where the ferrihydrite crystals exposed by ferritin degradation dissolve in the lysosomal fluid, and the reduced iron is transported back to the cytosol via DMT1 in hepatocytes, and by both DMT1 and Nramp1 in macrophages, prior to release into the blood or storage in ferritin.
中文翻译:

铁蛋白中铁的动员:新的步骤和细节
许多证据表明,储存在铁蛋白中的铁是通过溶酶体中的蛋白质降解而动员的,但对此过程的担忧一直存在,并且缺乏有关其机制的详细信息。在此处介绍的研究中,通过用放射性标记的铁预加载肝(HepG2)细胞来诱导59种铁标记的铁蛋白。将这些细胞置于含有去铁胺的培养基中会导致铁蛋白59 Fe的损失,但添加高浓度的还原剂或调节内部GSH的浓度却无法改变铁蛋白59的速率。铁释放。共聚焦显微镜检查表明,铁的剥夺增加了铁蛋白向溶酶体的运动,抑制了溶酶体的蛋白水解作用时,富集了铁。它也导致DMT1向溶酶体的快速移动,而这被bafilomycin抑制。从纯化的大鼠肝脏/脾铁蛋白中分离出的水铁矿晶体在pH 5和7下被从肝脏和J774a.1巨噬细胞获得的GSH,抗坏血酸盐,柠檬酸盐和溶酶体液溶解。DMT1 / Nramp2的抑制和Nramp1的siRNA敲低分别减少了59的转移铁从溶酶体到细胞质;小鼠中DMT1的肝细胞特异性敲除阻止了响应EPO处理的肝脏中铁的释放,但没有抑制溶酶体铁蛋白的降解。我们得出的结论是,铁蛋白-铁动员不是通过还原剂/螯合剂的细胞浓度变化而发生,而是通过铁蛋白和DMT1向溶酶体的协同运动而发生,溶酶体中铁蛋白降解所暴露的亚铁水合物晶体溶解在溶酶体液中,并且还原铁在释放入血液或在铁蛋白中储存之前,其通过肝细胞中的DMT1以及巨噬细胞中的DMT1和Nramp1运回到细胞质中。
更新日期:2017-12-04
中文翻译:

铁蛋白中铁的动员:新的步骤和细节
许多证据表明,储存在铁蛋白中的铁是通过溶酶体中的蛋白质降解而动员的,但对此过程的担忧一直存在,并且缺乏有关其机制的详细信息。在此处介绍的研究中,通过用放射性标记的铁预加载肝(HepG2)细胞来诱导59种铁标记的铁蛋白。将这些细胞置于含有去铁胺的培养基中会导致铁蛋白59 Fe的损失,但添加高浓度的还原剂或调节内部GSH的浓度却无法改变铁蛋白59的速率。铁释放。共聚焦显微镜检查表明,铁的剥夺增加了铁蛋白向溶酶体的运动,抑制了溶酶体的蛋白水解作用时,富集了铁。它也导致DMT1向溶酶体的快速移动,而这被bafilomycin抑制。从纯化的大鼠肝脏/脾铁蛋白中分离出的水铁矿晶体在pH 5和7下被从肝脏和J774a.1巨噬细胞获得的GSH,抗坏血酸盐,柠檬酸盐和溶酶体液溶解。DMT1 / Nramp2的抑制和Nramp1的siRNA敲低分别减少了59的转移铁从溶酶体到细胞质;小鼠中DMT1的肝细胞特异性敲除阻止了响应EPO处理的肝脏中铁的释放,但没有抑制溶酶体铁蛋白的降解。我们得出的结论是,铁蛋白-铁动员不是通过还原剂/螯合剂的细胞浓度变化而发生,而是通过铁蛋白和DMT1向溶酶体的协同运动而发生,溶酶体中铁蛋白降解所暴露的亚铁水合物晶体溶解在溶酶体液中,并且还原铁在释放入血液或在铁蛋白中储存之前,其通过肝细胞中的DMT1以及巨噬细胞中的DMT1和Nramp1运回到细胞质中。


























 京公网安备 11010802027423号
京公网安备 11010802027423号