当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and Biological Activity of 1,2,3-Triazolobenzodiazepine BET Bromodomain Inhibitors
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00389 Phillip P. Sharp 1, 2 , Jean-Marc Garnier 1, 2 , Tamas Hatfaludi 1, 2 , Zhen Xu 1, 2 , David Segal 1, 2 , Kate E. Jarman 1, 2 , Hélène Jousset 1, 2 , Alexandra Garnham 1, 2 , John T. Feutrill 3 , Anthony Cuzzupe 3 , Peter Hall 1, 2 , Scott Taylor 4 , Carl R. Walkley 4 , Dean Tyler 5 , Mark A. Dawson 5, 6, 7 , Peter Czabotar 1, 2 , Andrew F. Wilks 3 , Stefan Glaser 1, 2 , David C. S. Huang 1, 2 , Christopher J. Burns 1, 2, 8
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00389 Phillip P. Sharp 1, 2 , Jean-Marc Garnier 1, 2 , Tamas Hatfaludi 1, 2 , Zhen Xu 1, 2 , David Segal 1, 2 , Kate E. Jarman 1, 2 , Hélène Jousset 1, 2 , Alexandra Garnham 1, 2 , John T. Feutrill 3 , Anthony Cuzzupe 3 , Peter Hall 1, 2 , Scott Taylor 4 , Carl R. Walkley 4 , Dean Tyler 5 , Mark A. Dawson 5, 6, 7 , Peter Czabotar 1, 2 , Andrew F. Wilks 3 , Stefan Glaser 1, 2 , David C. S. Huang 1, 2 , Christopher J. Burns 1, 2, 8
Affiliation
|
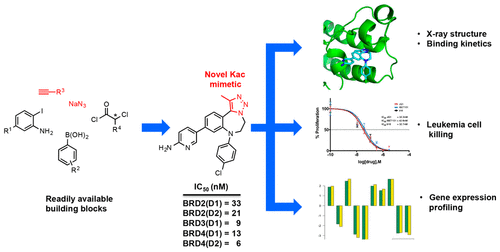
A number of diazepines are known to inhibit bromo- and extra-terminal domain (BET) proteins. Their BET inhibitory activity derives from the fusion of an acetyl-lysine mimetic heterocycle onto the diazepine framework. Herein we describe a straightforward, modular synthesis of novel 1,2,3-triazolobenzodiazepines and show that the 1,2,3-triazole acts as an effective acetyl-lysine mimetic heterocycle. Structure-based optimization of this series of compounds led to the development of potent BET bromodomain inhibitors with excellent activity against leukemic cells, concomitant with a reduction in c-MYC expression. These novel benzodiazepines therefore represent a promising class of therapeutic BET inhibitors.
中文翻译:

1,2,3-三唑并苯并二氮杂BET溴结构域抑制剂的设计,合成和生物活性
已知许多二氮杂卓可抑制溴末端和末端外域(BET)蛋白。它们的BET抑制活性源自乙酰赖氨酸模拟杂环在二氮杂骨架上的融合。在这里,我们描述了新颖的1,2,3-三唑并苯并二氮杂pine的简单,模块化合成,并表明1,2,3-三唑可作为有效的乙酰赖氨酸模拟杂环。该系列化合物的基于结构的优化导致开发出有效的BET溴结构域抑制剂,该抑制剂对白血病细胞具有出色的活性,并伴有c- MYC表达的降低。因此,这些新型苯并二氮杂pine代表了有前途的一类治疗性BET抑制剂。
更新日期:2017-12-01
中文翻译:

1,2,3-三唑并苯并二氮杂BET溴结构域抑制剂的设计,合成和生物活性
已知许多二氮杂卓可抑制溴末端和末端外域(BET)蛋白。它们的BET抑制活性源自乙酰赖氨酸模拟杂环在二氮杂骨架上的融合。在这里,我们描述了新颖的1,2,3-三唑并苯并二氮杂pine的简单,模块化合成,并表明1,2,3-三唑可作为有效的乙酰赖氨酸模拟杂环。该系列化合物的基于结构的优化导致开发出有效的BET溴结构域抑制剂,该抑制剂对白血病细胞具有出色的活性,并伴有c- MYC表达的降低。因此,这些新型苯并二氮杂pine代表了有前途的一类治疗性BET抑制剂。

























 京公网安备 11010802027423号
京公网安备 11010802027423号