当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Well-Defined Cationic N-[3-(Dimethylamino)propyl]methacrylamide Hydrochloride-Based (Co)polymers for siRNA Delivery
Biomacromolecules ( IF 6.2 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.biomac.7b01475 Pratyawadee Singhsa 1, 2 , Diana Diaz-Dussan 2 , Hathaikarn Manuspiya 1 , Ravin Narain 2
Biomacromolecules ( IF 6.2 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.biomac.7b01475 Pratyawadee Singhsa 1, 2 , Diana Diaz-Dussan 2 , Hathaikarn Manuspiya 1 , Ravin Narain 2
Affiliation
|
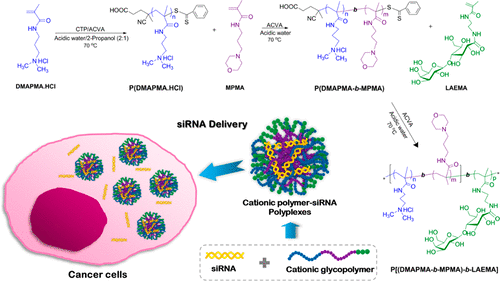
Cationic glycopolymers have shown to be excellent candidates for the fabrication of gene delivery devices due to their ability to electrostatically interact with negatively charged nucleic acids and the carbohydrate residues ensure enhanced stability and low toxicity of the polyplexes. The ability to engineer the polymers for optimized compositions, molecular weights, and architectures is critical in the design of effective gene delivery vehicles. Therefore, in this study, the aqueous reversible addition–fragmentation chain transfer polymerization (RAFT) was used to synthesize well-defined cationic glycopolymers with various cationic segments. For the preparation of cationic parts, N-[3-(dimethylamino)propyl]methacrylamide hydrochloride (DMAPMA·HCl), water-soluble methacrylamide monomer containing tertiary amine, was polymerized to produce DMAPMA·HCl homopolymer, which was then used as macroCTA in the block copolymerization with two other methacrylamide monomers containing different pendant groups, namely, 2-aminoethyl methacrylamide hydrochloride (AEMA) (with primary amine) and N-(3-aminopropyl) morpholine methacrylamide (MPMA) (with morpholine ring). In addition, statistical copolymers of DMAPMA.HCl with either AEMA or MPMA were also synthesized. All resulting cationic polymers were utilized as macroCTA for the RAFT copolymerization with 2-lactobionamidoethyl methacrylamide (LAEMA), which consists of the pendent galactose residues to achieve DMAPMA·HCl-based glycopolymers. From the in vitro cytotoxicity study, the cationic glycopolymers showed better cell viabilities than the corresponding cationic homopolymers. Furthermore, complexation of the cationic polymers with siRNA, cellular uptake of the resulting polyplexes, and gene knockdown efficiencies were evaluated. All cationic polymers/glycopolymers demonstrated good complexation ability with siRNA at low weight ratios. Among these cationic polymer-siRNA polyplexes, the polyplexes prepared from the two glycopolymers, P(DMAPMA65-b-LAEMA15) and P[(DMAPMA65-b-MPMA63)-b-LAEMA16], showed outstanding results in the cellular uptake, high EGFR knockdown, and low post-transfection toxicity, suggesting the great potential in siRNA delivery of these novel glycopolymers.
中文翻译:

定义明确的阳离子N- [3-(二甲氨基)丙基]甲基丙烯酰胺盐酸盐基(Co)聚合物,用于siRNA递送
由于阳离子糖聚合物与带负电荷的核酸发生静电相互作用的能力,因此已证明是制造基因传递装置的极佳候选者,而碳水化合物残基则确保了复合物的增强的稳定性和低毒性。对聚合物进行工程改造以使其具有最佳组成,分子量和结构的能力,对于设计有效的基因传递载体至关重要。因此,在这项研究中,使用了可逆的加成-断裂链转移水性聚合(RAFT)来合成具有各种阳离子链段的明确定义的阳离子糖聚合物。用于制备阳离子零件,N聚合含有叔胺的水溶性甲基丙烯酰胺单体-[3-(二甲氨基)丙基]甲基丙烯酰胺盐酸盐(DMAPMA·HCl),以生产DMAPMA·HCl均聚物,然后将其用作macroCTA与另外两种甲基丙烯酰胺单体进行嵌段共聚包含不同的侧基,即2-氨基乙基甲基丙烯酰胺盐酸盐(AEMA)(含伯胺)和N-(3-氨基丙基)吗啉甲基丙烯酰胺(MPMA)(带有吗啉环)。此外,还合成了DMAPMA.HCl与AEMA或MPMA的统计共聚物。所有得到的阳离子聚合物均用作macroCTA,用于与2-半乳糖氨基乙基甲基丙烯酰胺(LAEMA)共聚的RAFT共聚,该半乳糖残基由半乳糖残基组成,从而获得基于DMAPMA·HCl的糖聚合物。从体外细胞毒性研究来看,阳离子糖聚合物比相应的阳离子均聚物具有更好的细胞活力。此外,还评估了阳离子聚合物与siRNA的复合,细胞对所得多聚体的摄取以及基因敲除效率。在低重量比下,所有阳离子聚合物/糖聚合物均表现出与siRNA良好的络合能力。在这些阳离子聚合物-siRNA复合物中,65 - b -LAEMA 15)和P [(DMAPMA 65 - b -MPMA 63)-b -LAEMA 16 ]在细胞摄取,高EGFR敲低和低转染后毒性方面显示出优异的结果,表明在这些新型糖聚合物的siRNA递送。
更新日期:2017-12-20
中文翻译:

定义明确的阳离子N- [3-(二甲氨基)丙基]甲基丙烯酰胺盐酸盐基(Co)聚合物,用于siRNA递送
由于阳离子糖聚合物与带负电荷的核酸发生静电相互作用的能力,因此已证明是制造基因传递装置的极佳候选者,而碳水化合物残基则确保了复合物的增强的稳定性和低毒性。对聚合物进行工程改造以使其具有最佳组成,分子量和结构的能力,对于设计有效的基因传递载体至关重要。因此,在这项研究中,使用了可逆的加成-断裂链转移水性聚合(RAFT)来合成具有各种阳离子链段的明确定义的阳离子糖聚合物。用于制备阳离子零件,N聚合含有叔胺的水溶性甲基丙烯酰胺单体-[3-(二甲氨基)丙基]甲基丙烯酰胺盐酸盐(DMAPMA·HCl),以生产DMAPMA·HCl均聚物,然后将其用作macroCTA与另外两种甲基丙烯酰胺单体进行嵌段共聚包含不同的侧基,即2-氨基乙基甲基丙烯酰胺盐酸盐(AEMA)(含伯胺)和N-(3-氨基丙基)吗啉甲基丙烯酰胺(MPMA)(带有吗啉环)。此外,还合成了DMAPMA.HCl与AEMA或MPMA的统计共聚物。所有得到的阳离子聚合物均用作macroCTA,用于与2-半乳糖氨基乙基甲基丙烯酰胺(LAEMA)共聚的RAFT共聚,该半乳糖残基由半乳糖残基组成,从而获得基于DMAPMA·HCl的糖聚合物。从体外细胞毒性研究来看,阳离子糖聚合物比相应的阳离子均聚物具有更好的细胞活力。此外,还评估了阳离子聚合物与siRNA的复合,细胞对所得多聚体的摄取以及基因敲除效率。在低重量比下,所有阳离子聚合物/糖聚合物均表现出与siRNA良好的络合能力。在这些阳离子聚合物-siRNA复合物中,65 - b -LAEMA 15)和P [(DMAPMA 65 - b -MPMA 63)-b -LAEMA 16 ]在细胞摄取,高EGFR敲低和低转染后毒性方面显示出优异的结果,表明在这些新型糖聚合物的siRNA递送。


























 京公网安备 11010802027423号
京公网安备 11010802027423号