当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of tacrine-1,2,3-triazole derivatives as potent cholinesterase inhibitors†
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1039/c7md00457e Gaochan Wu 1, 2, 3, 4, 5 , Yun Gao 1, 2, 3, 4, 5 , Dongwei Kang 1, 2, 3, 4, 5 , Boshi Huang 1, 2, 3, 4, 5 , Zhipeng Huo 1, 2, 3, 4, 5 , Huiqing Liu 4, 5, 6, 7, 8 , Vasanthanathan Poongavanam 9, 10, 11, 12 , Peng Zhan 1, 2, 3, 4, 5 , Xinyong Liu 1, 2, 3, 4, 5
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1039/c7md00457e Gaochan Wu 1, 2, 3, 4, 5 , Yun Gao 1, 2, 3, 4, 5 , Dongwei Kang 1, 2, 3, 4, 5 , Boshi Huang 1, 2, 3, 4, 5 , Zhipeng Huo 1, 2, 3, 4, 5 , Huiqing Liu 4, 5, 6, 7, 8 , Vasanthanathan Poongavanam 9, 10, 11, 12 , Peng Zhan 1, 2, 3, 4, 5 , Xinyong Liu 1, 2, 3, 4, 5
Affiliation
|
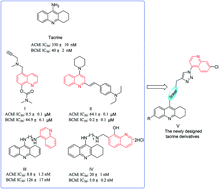
We report herein the design and synthesis of a series of 11 novel tacrine-1,2,3-triazole derivatives via a Cu(I)-catalyzed alkyne–azide 1,3-dipolar cycloaddition (CuAAC) reaction. The newly synthesized compounds were evaluated for their inhibition activity against Electrophorus electricus acetylcholinesterase (AChE) and horse serum butyrylcholinesterase (BChE) as potential drug targets for Alzheimer's disease (AD). Among the designed compounds, compound 8a2 exhibited potent inhibition against AChE and BChE with IC50 values of 4.89 μM and 3.61 μM, respectively. Further structure–activity relationship (SAR) and molecular modeling studies may provide valuable insights into the design of better tacrine-triazole analogues with potential therapeutic applications for AD.
中文翻译:

他克林-1,2,3-三唑衍生物作为有效胆碱酯酶抑制剂的设计,合成和生物学评估†
我们在此报告通过Cu(I)催化的炔-叠氮化物1,3-偶极环加成(CuAAC)反应设计和合成一系列11种新颖的他克林-1,2,3-三唑衍生物。评价了新合成的化合物对作为潜在的阿尔茨海默氏病(AD)药物靶点的电乙酰胆碱酯酶(AChE)和马血清丁酰胆碱酯酶(BChE)的抑制活性。在设计的化合物中,化合物8a2对AChE和BChE的抑制力为IC 50值分别为4.89μM和3.61μM。进一步的结构-活性关系(SAR)和分子模型研究可能为更好的他克林-三唑类似物的设计提供有价值的见解,并可能将其用于AD。
更新日期:2017-12-01
中文翻译:

他克林-1,2,3-三唑衍生物作为有效胆碱酯酶抑制剂的设计,合成和生物学评估†
我们在此报告通过Cu(I)催化的炔-叠氮化物1,3-偶极环加成(CuAAC)反应设计和合成一系列11种新颖的他克林-1,2,3-三唑衍生物。评价了新合成的化合物对作为潜在的阿尔茨海默氏病(AD)药物靶点的电乙酰胆碱酯酶(AChE)和马血清丁酰胆碱酯酶(BChE)的抑制活性。在设计的化合物中,化合物8a2对AChE和BChE的抑制力为IC 50值分别为4.89μM和3.61μM。进一步的结构-活性关系(SAR)和分子模型研究可能为更好的他克林-三唑类似物的设计提供有价值的见解,并可能将其用于AD。


























 京公网安备 11010802027423号
京公网安备 11010802027423号