当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Intracellularly Actuated Quantum Dot–Peptide–Doxorubicin Nanobioconjugates for Controlled Drug Delivery via the Endocytic Pathway
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00658 Ajmeeta Sangtani 1 , Eleonora Petryayeva 2 , Miao Wu 2 , Kimihiro Susumu 3 , Eunkeu Oh 3 , Alan L. Huston , Guillermo Lasarte-Aragones 4 , Igor L. Medintz , W. Russ Algar 2 , James B. Delehanty
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2017-12-21 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00658 Ajmeeta Sangtani 1 , Eleonora Petryayeva 2 , Miao Wu 2 , Kimihiro Susumu 3 , Eunkeu Oh 3 , Alan L. Huston , Guillermo Lasarte-Aragones 4 , Igor L. Medintz , W. Russ Algar 2 , James B. Delehanty
Affiliation
|
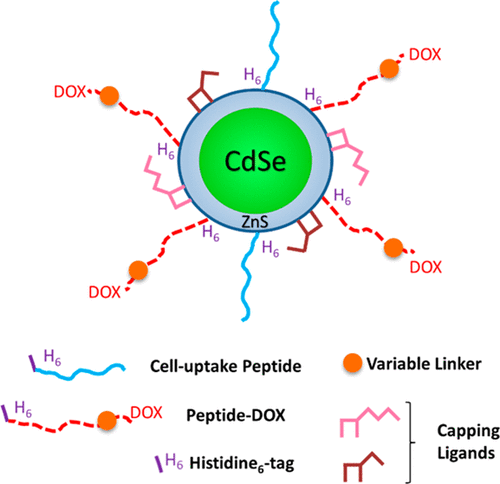
Nanoparticle (NP)-mediated drug delivery (NMDD) has emerged as a novel method to overcome the limitations of traditional systemic delivery of therapeutics, including the controlled release of the NP-associated drug cargo. Currently, our most advanced understanding of how to control NP-associated cargos is in the context of soft nanoparticles (e.g., liposomes), but less is known about controlling the release of cargos from the surface of hard NPs (e.g., gold NPs). Here we employ a semiconductor quantum dot (QD) as a prototypical hard NP platform and use intracellularly triggered actuation to achieve spatiotemporal control of drug release and modulation of drug efficacy. Conjugated to the QD are two peptides: (1) a cell-penetrating peptide (CPP) that facilitates uptake of the conjugate into the endocytic pathway and (2) a display peptide conjugated to doxorubicin (DOX) via three different linkages (ester, disulfide, and hydrazone) that are responsive to enzymatic cleavage, reducing conditions, and low pH, respectively. Formation of the QD–[peptide–DOX]–CPP complex is driven by self-assembly that allows control over both the ratio of each peptide species conjugated to the QD and the eventual drug dose delivered to cells. Förster resonance energy transfer assays confirmed successful assembly of the QD–peptide complexes and functionality of the linkages. Confocal microscopy was employed to visualize residence of the QD–[peptide–DOX]–CPP complexes in the endocytic pathway, and distinct differences in DOX localization were noted for the ester linkage, which showed clear signs of nuclear delivery versus the hydrazone, disulfide, and amide control. Finally, delivery of the QD–[peptide–DOX]–CPP conjugate resulted in cytotoxicity for the ester linkage that was comparable to free DOX. Attachment of DOX via the hydrazone linkage facilitated intermediary toxicity, while the disulfide and amide control linkages showed minimal toxicity. Our data demonstrate the utility of hard NP–peptide bioconjugates to function as multifunctional scaffolds for simultaneous control over cellular drug uptake and toxicity and the vital role played by the nature of the chemical linkage that appends the drug to the NP carrier.
中文翻译:

细胞内驱动的量子点-肽-阿霉素纳米生物共轭物通过内吞途径控制药物的传递
纳米粒子(NP)介导的药物传递(NMDD)已经成为克服传统系统疗法的局限性的新方法,其中包括与NP相关的药物货物的受控释放。当前,我们对如何控制与NP相关的货物的最先进的理解是在软纳米颗粒(例如脂质体)的背景下进行的,但对于控制货物从硬NP(例如金NP)表面的释放知之甚少。在这里,我们采用半导体量子点(QD)作为原型硬NP平台,并使用细胞内触发的致动来实现时空控制药物释放和调节药物功效。与QD结合的是两种肽:(1)促进共轭物进入内吞途径的细胞穿透肽(CPP),以及(2)通过三个不同的键(酯,二硫键和)与阿霉素(DOX)缀合的展示肽酶促裂解,还原条件和低pH值。QD- [肽-DOX] -CPP复合物的形成是由自组装驱动的,它可以控制与QD结合的每种肽的比率以及最终传递给细胞的药物剂量。Förster共振能量转移测定法证实了QD-肽复合物的成功组装和键的功能性。共聚焦显微镜用于观察QD- [肽-DOX] -CPP复合物在胞吞途径中的驻留,并且发现酯键的DOX定位存在明显差异,与,二硫化物和酰胺对照相比,它显示出明显的核传递迹象。最后,QD- [肽-DOX] -CPP偶联物的递送导致酯键的细胞毒性与游离的DOX相当。通过X键连接的DOX促进了中间毒性,而二硫键和酰胺控制键显示出最小的毒性。我们的数据表明,硬NP肽生物共轭物可用作多功能支架,可同时控制细胞对药物的吸收和毒性,以及将药物附加到NP载体上的化学键的性质起着至关重要的作用。QD- [肽-DOX] -CPP偶联物的递送导致酯键的细胞毒性可与游离DOX相比。通过X键连接的DOX促进了中间毒性,而二硫键和酰胺控制键显示出最小的毒性。我们的数据表明,硬NP肽生物共轭物可用作多功能支架,可同时控制细胞对药物的吸收和毒性,以及将药物附加到NP载体上的化学键的性质起着至关重要的作用。QD- [肽-DOX] -CPP偶联物的递送导致酯键的细胞毒性可与游离DOX相比。通过X键连接的DOX促进了中间毒性,而二硫键和酰胺控制键显示出最小的毒性。我们的数据表明,硬NP肽生物共轭物可用作多功能支架,可同时控制细胞对药物的吸收和毒性,以及将药物附加到NP载体上的化学键的性质起着至关重要的作用。
更新日期:2017-12-21
中文翻译:

细胞内驱动的量子点-肽-阿霉素纳米生物共轭物通过内吞途径控制药物的传递
纳米粒子(NP)介导的药物传递(NMDD)已经成为克服传统系统疗法的局限性的新方法,其中包括与NP相关的药物货物的受控释放。当前,我们对如何控制与NP相关的货物的最先进的理解是在软纳米颗粒(例如脂质体)的背景下进行的,但对于控制货物从硬NP(例如金NP)表面的释放知之甚少。在这里,我们采用半导体量子点(QD)作为原型硬NP平台,并使用细胞内触发的致动来实现时空控制药物释放和调节药物功效。与QD结合的是两种肽:(1)促进共轭物进入内吞途径的细胞穿透肽(CPP),以及(2)通过三个不同的键(酯,二硫键和)与阿霉素(DOX)缀合的展示肽酶促裂解,还原条件和低pH值。QD- [肽-DOX] -CPP复合物的形成是由自组装驱动的,它可以控制与QD结合的每种肽的比率以及最终传递给细胞的药物剂量。Förster共振能量转移测定法证实了QD-肽复合物的成功组装和键的功能性。共聚焦显微镜用于观察QD- [肽-DOX] -CPP复合物在胞吞途径中的驻留,并且发现酯键的DOX定位存在明显差异,与,二硫化物和酰胺对照相比,它显示出明显的核传递迹象。最后,QD- [肽-DOX] -CPP偶联物的递送导致酯键的细胞毒性与游离的DOX相当。通过X键连接的DOX促进了中间毒性,而二硫键和酰胺控制键显示出最小的毒性。我们的数据表明,硬NP肽生物共轭物可用作多功能支架,可同时控制细胞对药物的吸收和毒性,以及将药物附加到NP载体上的化学键的性质起着至关重要的作用。QD- [肽-DOX] -CPP偶联物的递送导致酯键的细胞毒性可与游离DOX相比。通过X键连接的DOX促进了中间毒性,而二硫键和酰胺控制键显示出最小的毒性。我们的数据表明,硬NP肽生物共轭物可用作多功能支架,可同时控制细胞对药物的吸收和毒性,以及将药物附加到NP载体上的化学键的性质起着至关重要的作用。QD- [肽-DOX] -CPP偶联物的递送导致酯键的细胞毒性可与游离DOX相比。通过X键连接的DOX促进了中间毒性,而二硫键和酰胺控制键显示出最小的毒性。我们的数据表明,硬NP肽生物共轭物可用作多功能支架,可同时控制细胞对药物的吸收和毒性,以及将药物附加到NP载体上的化学键的性质起着至关重要的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号